ELECTRONIC STRUCTURE OF ATOMS
... the light emitted must be quantized and appear as a line spectrum. Bohrs model was important since it quantized energy states for electrons. However, it only worked for atoms and ions with one electron. Since we know light has a particle nature, does matter have a wave nature? Louis de Broglie ...
... the light emitted must be quantized and appear as a line spectrum. Bohrs model was important since it quantized energy states for electrons. However, it only worked for atoms and ions with one electron. Since we know light has a particle nature, does matter have a wave nature? Louis de Broglie ...
Physics and the Quantum Mechanical Model
... Only way to know precise position of a subatomic particle is for a photon (light) to collide with it. The collision will change the velocity of the particle Therefore, we can’t know both at the exact same time. ...
... Only way to know precise position of a subatomic particle is for a photon (light) to collide with it. The collision will change the velocity of the particle Therefore, we can’t know both at the exact same time. ...
Waves & Oscillations Physics 42200 Spring 2013 Semester Matthew Jones
... • Matter particles are quantized “waves” in an underlying “field”. ...
... • Matter particles are quantized “waves” in an underlying “field”. ...
Atomic Structure
... Light is a wave…right? • Einstein’s interpretation of the photoelectric effect (1905) was that light is quantized in packets of set energy called photons. (He won the Nobel Prize for this.) • This meant that light had characteristics of particles! ...
... Light is a wave…right? • Einstein’s interpretation of the photoelectric effect (1905) was that light is quantized in packets of set energy called photons. (He won the Nobel Prize for this.) • This meant that light had characteristics of particles! ...
Phys202_Exam3_2006.doc
... 28. What is the interpretation of wave function of quantum mechanics? a. probability b. quantized relation c. relative number of occurrences d. ~ as a probability amplitude e. entropy ...
... 28. What is the interpretation of wave function of quantum mechanics? a. probability b. quantized relation c. relative number of occurrences d. ~ as a probability amplitude e. entropy ...
PPT
... The positions of the principal maxima occur at f = 0, 2p, 4p, ... where f is the phase between adjacent slits. q = 0, /d, 2/d, ... The intensity at the peak of a principal maximum goes as N2. 3 slits: Atot = 3A1 Itot = 9I1. N slits: IN = N2I1. Between two principal maxima there are N-1 zeros ...
... The positions of the principal maxima occur at f = 0, 2p, 4p, ... where f is the phase between adjacent slits. q = 0, /d, 2/d, ... The intensity at the peak of a principal maximum goes as N2. 3 slits: Atot = 3A1 Itot = 9I1. N slits: IN = N2I1. Between two principal maxima there are N-1 zeros ...
1AMQ, Part II Quantum Mechanics
... from a slit, one photon at a time. The experiment shows that individual particles of light gradually build up the diffraction pattern predicted by the classical wave theory. The wave pattern describes the probability of detecting a photon at that point. The wave pattern measures the probability dete ...
... from a slit, one photon at a time. The experiment shows that individual particles of light gradually build up the diffraction pattern predicted by the classical wave theory. The wave pattern describes the probability of detecting a photon at that point. The wave pattern measures the probability dete ...
Wave Theory
... Although the double-slit experiment is now often referred to in the context of quantum mechanics, it is generally thought to have been first performed by the English scientist Thomas Young in the year 1801 in an attempt to resolve the question of whether light was composed of particles (Newton's "co ...
... Although the double-slit experiment is now often referred to in the context of quantum mechanics, it is generally thought to have been first performed by the English scientist Thomas Young in the year 1801 in an attempt to resolve the question of whether light was composed of particles (Newton's "co ...
Otto Stern and the discovery of space quantization
... moment. Now there is nothing in physics to suggest that these magnetic moments and angular moments would line up in a magnetic field in any coherent fashion. Because the angular moments could point in any direction, there would be a big concentration equatorially around the zero projection. The resu ...
... moment. Now there is nothing in physics to suggest that these magnetic moments and angular moments would line up in a magnetic field in any coherent fashion. Because the angular moments could point in any direction, there would be a big concentration equatorially around the zero projection. The resu ...
Chapter 2 (Particle Properties of Waves)
... The spatial coordinate of any point of constant phase travels in the +x direction when /k is positive, and in the -x direction when /k is negative. In other words, waves travel to the right when /k is positive, and to the left when /k is negative. Thus, the signs of and k tell the direction of ...
... The spatial coordinate of any point of constant phase travels in the +x direction when /k is positive, and in the -x direction when /k is negative. In other words, waves travel to the right when /k is positive, and to the left when /k is negative. Thus, the signs of and k tell the direction of ...
Atomic Theory
... All matter is composed of extremely small particles called atoms. All atoms of a given element are identical. Atoms of a specific element are different from those of any other element. Atoms cannot be created, destroyed, or divided into smaller particles. Different atoms combine in simple whole-numb ...
... All matter is composed of extremely small particles called atoms. All atoms of a given element are identical. Atoms of a specific element are different from those of any other element. Atoms cannot be created, destroyed, or divided into smaller particles. Different atoms combine in simple whole-numb ...
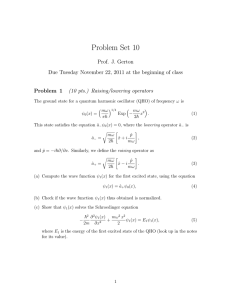
Problem Set 10
... components of the wavefunction? Why? (b) Write down the wave function for x > 0. Here, are there left- and right-moving components of the wavefunction? Why? (c) Write down the boundary conditions at x = 0 and solve for the amplitude coefficients of the reflected and transmitted waves, in terms of th ...
... components of the wavefunction? Why? (b) Write down the wave function for x > 0. Here, are there left- and right-moving components of the wavefunction? Why? (c) Write down the boundary conditions at x = 0 and solve for the amplitude coefficients of the reflected and transmitted waves, in terms of th ...