Announcement Station #2 Stars Lecture 9 Basic Physics The Laws
... • Two fermions of the same type cannot occupy the same quantum state at the same time. (This principle does not apply to bosons.) • How is the exclusion principle important to our ...
... • Two fermions of the same type cannot occupy the same quantum state at the same time. (This principle does not apply to bosons.) • How is the exclusion principle important to our ...
Fundamentals of Particle Physics
... • LIGO detected two black holes each around 30 times the mass of our sun orbiting each other with a frequency rising up to 250 times a second until they eventually collided ...
... • LIGO detected two black holes each around 30 times the mass of our sun orbiting each other with a frequency rising up to 250 times a second until they eventually collided ...
Quantum Measurements PHYSICS COLLOQUIUM Klaus Mølmer
... systems, and even today there is no, commonly agreed upon, understanding of the quantum measurement problem. The experimental situation and hence the subjects of theoretical investigations have, however, been considerably refined since the early days of quantum mechanics. Without claiming a solution ...
... systems, and even today there is no, commonly agreed upon, understanding of the quantum measurement problem. The experimental situation and hence the subjects of theoretical investigations have, however, been considerably refined since the early days of quantum mechanics. Without claiming a solution ...
Why is this a problem?
... This worked at longer wavelengths but deviates badly at short ones. This problem became known as the ultraviolet catastrophe and was one of the many effects classical physics couldn’t explain. ...
... This worked at longer wavelengths but deviates badly at short ones. This problem became known as the ultraviolet catastrophe and was one of the many effects classical physics couldn’t explain. ...
5.4 Quantum Devices Energy Levels in a Single Quantum Well
... Energy Bands in Multiple Quantum Wells Since single atoms may also be described as SQWs (for one electron you just have the hydrogen atom type with a Coulomb potential), we must expect that the wave function of the electrons start to overlap as soon as the single SQWs in the MQW structure are close ...
... Energy Bands in Multiple Quantum Wells Since single atoms may also be described as SQWs (for one electron you just have the hydrogen atom type with a Coulomb potential), we must expect that the wave function of the electrons start to overlap as soon as the single SQWs in the MQW structure are close ...
2008 Term 1 No 4
... http://physicsworld.com/cws/article/news/31763 While a single electron will behave as a purely quantum entity, the mere presence of another electron is enough to cause the electron to make the transition from quantum to classical behaviour -- according to an international team of physicists who have ...
... http://physicsworld.com/cws/article/news/31763 While a single electron will behave as a purely quantum entity, the mere presence of another electron is enough to cause the electron to make the transition from quantum to classical behaviour -- according to an international team of physicists who have ...
Homework Set 1
... atom. (This formula foreshadows the fact that, in general, the ground state of any system is the most in need of a quantum description.) c. Taking λ/r ≤ 0.1 as the (arbitrary) cut-off when classical mechanics begins to be valid as Bohr’s quantum number n increases, calculate the lowest (smallest n) ...
... atom. (This formula foreshadows the fact that, in general, the ground state of any system is the most in need of a quantum description.) c. Taking λ/r ≤ 0.1 as the (arbitrary) cut-off when classical mechanics begins to be valid as Bohr’s quantum number n increases, calculate the lowest (smallest n) ...
pptx
... How can the photon “know” when it encounters BS1 whether one or two paths are open? (whether we’re conducting Experiment One or Experiment Two?) What if the photon encounters BS1 while we are conducting Experiment One? - There is only one path to the second beam splitter. - It must “choose” to take ...
... How can the photon “know” when it encounters BS1 whether one or two paths are open? (whether we’re conducting Experiment One or Experiment Two?) What if the photon encounters BS1 while we are conducting Experiment One? - There is only one path to the second beam splitter. - It must “choose” to take ...
Chapter 41 Wave Mechanics 41.1 De Broglie Waves
... the wave extends over many cycles. But if a matter wave is spread out in space, the position of the particle is poorly defined. Thus to reduce the uncertainty in position of the particle, ∆x, one can propose many wave length to form a reasonably well-localized wave-packet. ...
... the wave extends over many cycles. But if a matter wave is spread out in space, the position of the particle is poorly defined. Thus to reduce the uncertainty in position of the particle, ∆x, one can propose many wave length to form a reasonably well-localized wave-packet. ...
January 2005
... A simple model of a polymer consists of N + 1 monomers connected by bonds of fixed length a. Le the chain be confined to a plane, so that the position of each monomer i is a vector ri in two dimensions; alternatively the polymer can be described by angles θi that measure the orientation of the bond ...
... A simple model of a polymer consists of N + 1 monomers connected by bonds of fixed length a. Le the chain be confined to a plane, so that the position of each monomer i is a vector ri in two dimensions; alternatively the polymer can be described by angles θi that measure the orientation of the bond ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 8. Write the Hamiltonian operator for the H2+ molecular ion in atomic units defining each term involved in it. 9. Explain the principle of mutual exclusion with an example. 10. Identify the point groups for the following molecules: (a) Br2 (b) CH3Br (c) [Co(NH3)6]3+ d) IF5 PART-B ANSWER ANY EIGHT QU ...
... 8. Write the Hamiltonian operator for the H2+ molecular ion in atomic units defining each term involved in it. 9. Explain the principle of mutual exclusion with an example. 10. Identify the point groups for the following molecules: (a) Br2 (b) CH3Br (c) [Co(NH3)6]3+ d) IF5 PART-B ANSWER ANY EIGHT QU ...
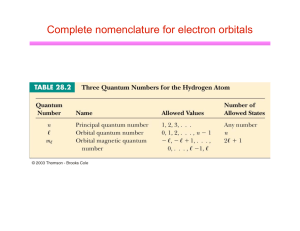
Complete nomenclature for electron orbitals
... l It pictures the electrons as orbiting the nucleus in circular (or elliptical orbitals) l But in fact the only reality is |y|2, the square of the wavefunction, which gives the probability of the electron to be in a given place at a given time l Electron is not confined to any particular orbital dis ...
... l It pictures the electrons as orbiting the nucleus in circular (or elliptical orbitals) l But in fact the only reality is |y|2, the square of the wavefunction, which gives the probability of the electron to be in a given place at a given time l Electron is not confined to any particular orbital dis ...
Electrons and Photons
... • This can be explained by the movement of electrons! • We know from middle school that atoms have “layers” of electrons called energy levels. • Each energy level has electrons with a certain amount of energy in them that matches the level. • When the electrons change levels, they have to gain or lo ...
... • This can be explained by the movement of electrons! • We know from middle school that atoms have “layers” of electrons called energy levels. • Each energy level has electrons with a certain amount of energy in them that matches the level. • When the electrons change levels, they have to gain or lo ...
SPH4U Modern Plans
... Unit 5 Day 5: Perimeter Video 1. Video from the Perimeter Institute – overview of modern physics. 2. Continue to work on the Photoelectric Effect Lab Unit 5 Day 6: Fun Physics 1. Class period to work on the last assignment in the computer lab. 2. Work on Fun Physics Assignment F1.1, F2.1, F3.1, F3.2 ...
... Unit 5 Day 5: Perimeter Video 1. Video from the Perimeter Institute – overview of modern physics. 2. Continue to work on the Photoelectric Effect Lab Unit 5 Day 6: Fun Physics 1. Class period to work on the last assignment in the computer lab. 2. Work on Fun Physics Assignment F1.1, F2.1, F3.1, F3.2 ...