Diffusion of Individual Atoms
... In a scientific first, a team of researchers from Macquarie University and the University of Vienna have developed a new technique to measure molecular properties – forming the basis for improvements in scientific instruments like telescopes, and with the potential to speed up the development of pha ...
... In a scientific first, a team of researchers from Macquarie University and the University of Vienna have developed a new technique to measure molecular properties – forming the basis for improvements in scientific instruments like telescopes, and with the potential to speed up the development of pha ...
lecture 11 (zipped power point)
... h is a proportional constant, called the Planck constant, experimentally determined to be: h = 6.626 x 10-34 Js (later) Momentum of photon, p = E /c = h /l This relation is obtained from SR relationship E2 = p2c2 + (m0c2)2, for which the mass of a photon is zero. {n,l} ...
... h is a proportional constant, called the Planck constant, experimentally determined to be: h = 6.626 x 10-34 Js (later) Momentum of photon, p = E /c = h /l This relation is obtained from SR relationship E2 = p2c2 + (m0c2)2, for which the mass of a photon is zero. {n,l} ...
particle in a box the uncertainty principle
... Planck's constant is so small that we never encounter the uncertainty principle in Newtonian mechanics… …but its consequences are manifested in materials we constantly use in everyday life! You’ll hear about it repeatedly in this course. *To placate his critics and get his uncertainty principle pape ...
... Planck's constant is so small that we never encounter the uncertainty principle in Newtonian mechanics… …but its consequences are manifested in materials we constantly use in everyday life! You’ll hear about it repeatedly in this course. *To placate his critics and get his uncertainty principle pape ...
Chapter 7 Quantum Theory of the Atom
... One property of waves is that they can be diffracted—that is, they spread out when they encounter an obstacle about the size of the wavelength. In 1801, Thomas Young, a British physicist, showed that light could be diffracted. By the early 1900s, the wave theory of light was well established. ...
... One property of waves is that they can be diffracted—that is, they spread out when they encounter an obstacle about the size of the wavelength. In 1801, Thomas Young, a British physicist, showed that light could be diffracted. By the early 1900s, the wave theory of light was well established. ...
Exercise Sheet 1 to Particle Physics I
... 1) Use the Particle Data Group (PDG) webpage (or other sources of information) to express the following quantities in the elementary particle physics natural units (i.e. in proper eV units using h̄ = c = 1): atomic radius (1 Å), nucleon radius (1 fm = typical size of atomic nuclei) classic electron ...
... 1) Use the Particle Data Group (PDG) webpage (or other sources of information) to express the following quantities in the elementary particle physics natural units (i.e. in proper eV units using h̄ = c = 1): atomic radius (1 Å), nucleon radius (1 fm = typical size of atomic nuclei) classic electron ...
Bohmian Mechanics
... the best tested predictions in the whole of science and nearly all of modern technology is based on our knowledge of quantum mechanics. Finally, when one listens to physicists, they speak very much as if there were something out there that one can talk about: particles are sent in this or that direc ...
... the best tested predictions in the whole of science and nearly all of modern technology is based on our knowledge of quantum mechanics. Finally, when one listens to physicists, they speak very much as if there were something out there that one can talk about: particles are sent in this or that direc ...
mp2b-16 honors
... Bohr – Planetary model A. What is Bohr's model for the atom? B. What does it look like? C. Where are the electons?! D. What was Bohr's explanation for what happens in a gas discharge tube? E. What are the good points about Bohr's atom? F. What is its weak point? ...
... Bohr – Planetary model A. What is Bohr's model for the atom? B. What does it look like? C. Where are the electons?! D. What was Bohr's explanation for what happens in a gas discharge tube? E. What are the good points about Bohr's atom? F. What is its weak point? ...
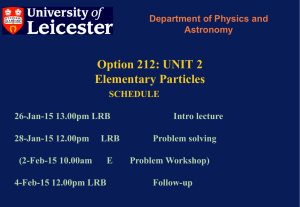
212 Particle Physics Lecture 1 - X-ray and Observational Astronomy
... http://superstringtheory.com/experm/index.html ...
... http://superstringtheory.com/experm/index.html ...
Principle of Least Action
... • Firstly, it’s elegant. In fact, it’s not just elegant: it’s completely gorgeous. In a way that theoretical physics should be, and usually is, and in a way that the old Newtonian mechanics really isn’t. • Secondly, it’s more powerful. It gives new methods to solve hard problems in a fairly straight ...
... • Firstly, it’s elegant. In fact, it’s not just elegant: it’s completely gorgeous. In a way that theoretical physics should be, and usually is, and in a way that the old Newtonian mechanics really isn’t. • Secondly, it’s more powerful. It gives new methods to solve hard problems in a fairly straight ...
Department of Physical Sciences (Physics)
... (i) Explain what is meant by the Photoelectric Effect. [2 marks] (ii) Describe briefly the apparatus used to study this effect and discuss the main experimental observations making reference to appropriate graphs of the results. Use these graphs to explain what is meant by: (a) prompt emission (b) t ...
... (i) Explain what is meant by the Photoelectric Effect. [2 marks] (ii) Describe briefly the apparatus used to study this effect and discuss the main experimental observations making reference to appropriate graphs of the results. Use these graphs to explain what is meant by: (a) prompt emission (b) t ...
Genovese_cern
... extracting QG effects from a limited (uncontrollable) observational sample affected by various propagation effects. ...
... extracting QG effects from a limited (uncontrollable) observational sample affected by various propagation effects. ...
Phy107Fall06Lect15 - UW High Energy Physics
... – He used the waves to form an interference pattern and calculated the wavelength – From v = f , v was found – v was very close to 3 x 108 m/s, the known speed of light • This provided evidence in support of Maxwell’s theory • This idea still used today measure wavelengths when studying stars Phy10 ...
... – He used the waves to form an interference pattern and calculated the wavelength – From v = f , v was found – v was very close to 3 x 108 m/s, the known speed of light • This provided evidence in support of Maxwell’s theory • This idea still used today measure wavelengths when studying stars Phy10 ...
SOLID-STATE PHYSICS 3, Winter 2008 O. Entin-Wohlman Conductivity and conductance
... (denoted “1”) to another (denoted “2”), by a diffusion process. The electron can take many paths between 1 and 2. In a classical calculation, we sum all over all the probabilities of the various paths; in quantum mechanics, we sum over the amplitudes of the various paths, and only then compute the t ...
... (denoted “1”) to another (denoted “2”), by a diffusion process. The electron can take many paths between 1 and 2. In a classical calculation, we sum all over all the probabilities of the various paths; in quantum mechanics, we sum over the amplitudes of the various paths, and only then compute the t ...
PPT | 299.77 KB - Joint Quantum Institute
... spurs the prospective integration of photonics and electronics. The JQI switch can steer a beam of light from one direction to another in only 120 ps using only about 90 attojoules of input power. At the wavelength used, in the near infrared (921 nm), this amounts to about 140 photons. In the PFC-su ...
... spurs the prospective integration of photonics and electronics. The JQI switch can steer a beam of light from one direction to another in only 120 ps using only about 90 attojoules of input power. At the wavelength used, in the near infrared (921 nm), this amounts to about 140 photons. In the PFC-su ...
Early Quantum Theory and Models of the Atom
... 1. Photoelectric effect 2. Move an electron to an excited state 3. Photon can be scattered resulting in lower frequency (energy) photon – called the Compton Effect ...
... 1. Photoelectric effect 2. Move an electron to an excited state 3. Photon can be scattered resulting in lower frequency (energy) photon – called the Compton Effect ...

![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/001036078_1-1a4f17b9367db590f7dcb987ef21bbe6-300x300.png)