Maths Makes Waves - people.bath.ac.uk
... But .. waves, and their mathematics, have a long history! Musical sounds: the first man made waves Greeks observed that some musical notes from a stringed instrument sound better when played together than others The octave C to C ...
... But .. waves, and their mathematics, have a long history! Musical sounds: the first man made waves Greeks observed that some musical notes from a stringed instrument sound better when played together than others The octave C to C ...
down - Display Materials Lab.
... 3.1 Physical meaning of wave function Postulate 1 : The state of a quantum mechanical system is completely specified by a wave function Ψ(x,t). The probability that a particle will be found at time t0 in a spatial interval of width dx centered at x0 is given by Ψ*(x0,t0)Ψ(x0,t0)dx. Meaning of wave ...
... 3.1 Physical meaning of wave function Postulate 1 : The state of a quantum mechanical system is completely specified by a wave function Ψ(x,t). The probability that a particle will be found at time t0 in a spatial interval of width dx centered at x0 is given by Ψ*(x0,t0)Ψ(x0,t0)dx. Meaning of wave ...
The Nature of Light: The Speed of Light in Gelatin and Wave
... through space in straight lines at a constant speed like harmless, invisible bullets shot from a gun. It wasn’t until the mid 20th century, after the formulation of the theory of quantum physics, that several experiments confirmed that light in fact behaves as both a particle AND a wave. These exper ...
... through space in straight lines at a constant speed like harmless, invisible bullets shot from a gun. It wasn’t until the mid 20th century, after the formulation of the theory of quantum physics, that several experiments confirmed that light in fact behaves as both a particle AND a wave. These exper ...
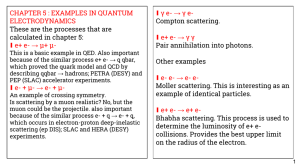
CHAPTER 5 : EXAMPLES IN QUANTUM γ e- → γ e- ∎ ELECTRODYNAMICS
... Substitute Mandelstam variables (See page 156) ...
... Substitute Mandelstam variables (See page 156) ...
Bell`s Inequality - weylmann.com
... To rephrase Einstein’s original thought experiment, the EPR effect involves an unstable, spin-zero particle which spontaneously decays into two spin-1/2 particles that then fly away from each other. Quantum physics says that initially each daughter particle is in a superposition of spin eigenstates, ...
... To rephrase Einstein’s original thought experiment, the EPR effect involves an unstable, spin-zero particle which spontaneously decays into two spin-1/2 particles that then fly away from each other. Quantum physics says that initially each daughter particle is in a superposition of spin eigenstates, ...
Introduction to Statistical Issues in Particle Physics - SLAC
... (higher-order calculations in perturbation theory, or the fragmentation of quarks into other particles). Furthermore the measurement and reconstruction process that the detector does for the particles is not completely accurate or completely efficient. The translation from knowing the distributions ...
... (higher-order calculations in perturbation theory, or the fragmentation of quarks into other particles). Furthermore the measurement and reconstruction process that the detector does for the particles is not completely accurate or completely efficient. The translation from knowing the distributions ...
here - Foundations of Physics 2013
... In 252 branches (25%) she will see half up, half down. In 10 branches (1%) she will see nine up, one down (which should be most likely, happening about 40% of the time). In general, most branches don’t exhibit Born Rule statistics. ...
... In 252 branches (25%) she will see half up, half down. In 10 branches (1%) she will see nine up, one down (which should be most likely, happening about 40% of the time). In general, most branches don’t exhibit Born Rule statistics. ...
Indiana University Physics P301: Modern Physics Review Problems
... (c) Evaluate the probability that the electron can be found in the angular region 0 < θ < π/4. [You can do the integral just over θ if you want, but be sure to also evaluate the normalization integral in this case.] (d) Moving from hydrogen to helium, can both electrons in the ground state of the at ...
... (c) Evaluate the probability that the electron can be found in the angular region 0 < θ < π/4. [You can do the integral just over θ if you want, but be sure to also evaluate the normalization integral in this case.] (d) Moving from hydrogen to helium, can both electrons in the ground state of the at ...
1 eV - Nikhef
... * The bunches of electrons excite microwaves in the output cavity 3 of the klystron. * The microwaves flow into the waveguide 4, which transports them to the accelerator. * The electrons are absorbed in the beam stop 5. ...
... * The bunches of electrons excite microwaves in the output cavity 3 of the klystron. * The microwaves flow into the waveguide 4, which transports them to the accelerator. * The electrons are absorbed in the beam stop 5. ...
CHAPTER 5
... 2. An electron may move from one discrete energy level (orbit) to another, but to do so energy is emitted or absorbed 3. An electron moves in a spherical orbit around the nucleus -If e- are in quantized energy states, then ∆E of states can have only certain values -This explains sharp line spectra ( ...
... 2. An electron may move from one discrete energy level (orbit) to another, but to do so energy is emitted or absorbed 3. An electron moves in a spherical orbit around the nucleus -If e- are in quantized energy states, then ∆E of states can have only certain values -This explains sharp line spectra ( ...
Particle Consistency of Microscopic and Macroscopic
... instability of any two adjacent atoms; Secondly, C. F. du Fay in 1733 showed a two-fluid theory of vitreous and resinous electricity, but in 1839 Michael Faraday believed the division of static electricity, current electricity, and bioelectricity to be only a consequence of the behavior of a single ...
... instability of any two adjacent atoms; Secondly, C. F. du Fay in 1733 showed a two-fluid theory of vitreous and resinous electricity, but in 1839 Michael Faraday believed the division of static electricity, current electricity, and bioelectricity to be only a consequence of the behavior of a single ...
Probing the Orbital Energy of an Electron in an Atom
... position, r0, where its kinetic energy is zero, Bills contradicts accepted quantum mechanical ideas regarding the behavior of electrons in atoms. The wave functions of atomic electrons are not eigenfunctions of the position, momentum, kinetic energy or potential energy operators. Consequently, accor ...
... position, r0, where its kinetic energy is zero, Bills contradicts accepted quantum mechanical ideas regarding the behavior of electrons in atoms. The wave functions of atomic electrons are not eigenfunctions of the position, momentum, kinetic energy or potential energy operators. Consequently, accor ...
ptt-file - Parmenides Foundation
... Need to include clock mechanism in the Hamiltonian. The system ‘fuses’ with the clock and changes its behaviour. Also We cannot make ...
... Need to include clock mechanism in the Hamiltonian. The system ‘fuses’ with the clock and changes its behaviour. Also We cannot make ...
P301_2010_week5
... •Identify and provide BRIEF descriptions of 3 important experimental results that came out of the study of electric currents in vacuum tubes or such tubes back-filled with dilute gas. •Provide an annotated sketch showing the essential elements of the apparatus used by Millikan to quantify individual ...
... •Identify and provide BRIEF descriptions of 3 important experimental results that came out of the study of electric currents in vacuum tubes or such tubes back-filled with dilute gas. •Provide an annotated sketch showing the essential elements of the apparatus used by Millikan to quantify individual ...
energy - Edublogs
... wavelengths of the spectral lines. 2. Since each element emits only certain wavelengths, the gas can be identified. ...
... wavelengths of the spectral lines. 2. Since each element emits only certain wavelengths, the gas can be identified. ...
Lecture notes, part 2
... is its average or mean value in the usual probability sense. In other words, it is a weighted average with |ψ|2 being the weight. In quantum mechanics this is generally called the expectation value and is denoted by angular brackets h·i. Usually we break |ψ|2 up, since multiplication is commutative, ...
... is its average or mean value in the usual probability sense. In other words, it is a weighted average with |ψ|2 being the weight. In quantum mechanics this is generally called the expectation value and is denoted by angular brackets h·i. Usually we break |ψ|2 up, since multiplication is commutative, ...
single
... logical basis for such a distinction. ‘Discovering’ a particle means observing certain effects which are accepted as proof of its existence.” A. S. Eddington, Fundamental Theory, (Cambridge University Press., Cambridge, 1942) pp. 30-31. ...
... logical basis for such a distinction. ‘Discovering’ a particle means observing certain effects which are accepted as proof of its existence.” A. S. Eddington, Fundamental Theory, (Cambridge University Press., Cambridge, 1942) pp. 30-31. ...