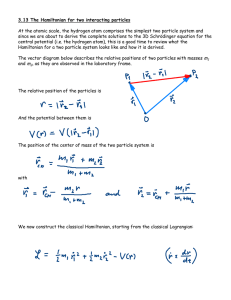
Document
... There are electrons with measured charge and mass There are positive charge to make the atom electrical neutral The size of atom is known to be about 10-10 m in radius ...
... There are electrons with measured charge and mass There are positive charge to make the atom electrical neutral The size of atom is known to be about 10-10 m in radius ...
power point notes
... Discovered that atoms contain negatively charged particles called “corpuscles” later called electrons ...
... Discovered that atoms contain negatively charged particles called “corpuscles” later called electrons ...
This Week Final Exam Marks on the Web
... atom – a positively-charged “pudding” with negatively-charged electron “plums” embedded in it (“plum pudding” model). This was ruled out by Rutherford following experiments by Geiger+Marsden... Monday, March 27, 2006 ...
... atom – a positively-charged “pudding” with negatively-charged electron “plums” embedded in it (“plum pudding” model). This was ruled out by Rutherford following experiments by Geiger+Marsden... Monday, March 27, 2006 ...
CHAPTER 4: Structure of the Atom
... The number of atoms across the thin gold layer of 6 × 10−7 m: ...
... The number of atoms across the thin gold layer of 6 × 10−7 m: ...
Geiger–Marsden experiment
The Geiger–Marsden experiment(s) (also called the Rutherford gold foil experiment) were a landmark series of experiments by which scientists discovered that every atom contains a nucleus where its positive charge and most of its mass are concentrated. They deduced this by measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The experiments were performed between 1908 and 1913 by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester.