The Quantum Mechanical Model
... Louis de Broglie (electron has wave properties) Erwin Schrodinger (mathematical equations using probability, quantum numbers) ...
... Louis de Broglie (electron has wave properties) Erwin Schrodinger (mathematical equations using probability, quantum numbers) ...
3.1 The correspondence principle
... The calculated energy Eigenvalues En and Eigenvectors fn define how the quantum mechanical system evolves in time: Let for t = 0 the system be in a state ...
... The calculated energy Eigenvalues En and Eigenvectors fn define how the quantum mechanical system evolves in time: Let for t = 0 the system be in a state ...
x - Piazza
... Time-Independent Schrödinger Wave Equation The potential in many cases will not depend explicitly on time: V = V(x). The Schrödinger equation’s dependence on time and position can then be separated. Let: ...
... Time-Independent Schrödinger Wave Equation The potential in many cases will not depend explicitly on time: V = V(x). The Schrödinger equation’s dependence on time and position can then be separated. Let: ...
... indistinguishability. In quantum theory, one may not, as in classical theory, specify both velocity and position exactly. Instead, the maximum specification of a physical system is given by the wave function 1/;. It is also termed the probability amplitude because its square, 11/;1 2, is the probabi ...
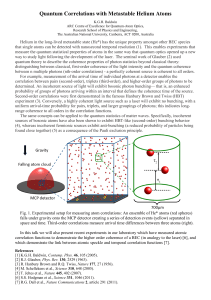
Quantum Correlations with Metastable Helium Atoms
... correlation between pairs (second-order), triplets (third-order), and higher-order groups of photons to be determined. An incoherent source of light will exhibit bosonic photon bunching— that is, an enhanced probability of groups of photons arriving within an interval that defines the coherence time ...
... correlation between pairs (second-order), triplets (third-order), and higher-order groups of photons to be determined. An incoherent source of light will exhibit bosonic photon bunching— that is, an enhanced probability of groups of photons arriving within an interval that defines the coherence time ...
Solutions Fall 2004 Due 5:01 PM, Tuesday 2004/10/12
... reflected or refracted off of the various planes making up the crystal lattice being studied. This type of interference depends on the wavelength of the beam, and the interaction between the beam constituents and the atoms that make up the crystal. Just like photons, protons and neutrons do have wav ...
... reflected or refracted off of the various planes making up the crystal lattice being studied. This type of interference depends on the wavelength of the beam, and the interaction between the beam constituents and the atoms that make up the crystal. Just like photons, protons and neutrons do have wav ...
Slides from lecture 4.
... Now put 18 people (electrons) in the auditorium (atom). Note that no two people (electrons) can occupy the same seat (state)! So, when one row is filled, a new row is started. This is a fundamental property of quantum mechanics, i.e., no two electrons in an atom can exist in the same state. It is ca ...
... Now put 18 people (electrons) in the auditorium (atom). Note that no two people (electrons) can occupy the same seat (state)! So, when one row is filled, a new row is started. This is a fundamental property of quantum mechanics, i.e., no two electrons in an atom can exist in the same state. It is ca ...
Chap 6.
... greater than its maximum observable component in any direction, namely `h̄. The quantum-mechanical behavior of the angular momentum and its components can be represented by a vector model, p illustrated in Fig. 5. The angular momentum vector L, with magnitude `(` + 1)h̄, can be pictured as precessi ...
... greater than its maximum observable component in any direction, namely `h̄. The quantum-mechanical behavior of the angular momentum and its components can be represented by a vector model, p illustrated in Fig. 5. The angular momentum vector L, with magnitude `(` + 1)h̄, can be pictured as precessi ...
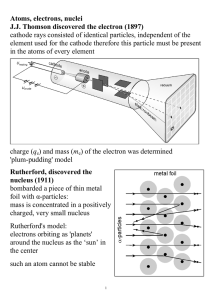
Atom is a basic unit of matter that consists of a nucleus
... Hydrogen-1 (one proton + one electron) is the simplest form of atoms, and not surprisingly, our quantum mechanical understanding of atoms evolved with the understanding of this species. In 1913, physicist Niels Bohr suggested that the electrons were confined into clearly defined, quantized orbits, a ...
... Hydrogen-1 (one proton + one electron) is the simplest form of atoms, and not surprisingly, our quantum mechanical understanding of atoms evolved with the understanding of this species. In 1913, physicist Niels Bohr suggested that the electrons were confined into clearly defined, quantized orbits, a ...
Atomic Spectra
... E RH 2 2 nl nh where RH is the Rydberg constant for hydrogen (= 2.179 × 10-18 J = 13.61 eV = 109677 cm-1); nl nh , are integers (l for lower lever and h for higher lever). ...
... E RH 2 2 nl nh where RH is the Rydberg constant for hydrogen (= 2.179 × 10-18 J = 13.61 eV = 109677 cm-1); nl nh , are integers (l for lower lever and h for higher lever). ...
On the Quantum Aspects of Geophysics
... is small, as well, due to the fact that the probability of observing this fast particle at x ≈ 0 is small. As x becomes larger the wavelength and the amplitude of the particle’s wave gets larger, as well, due to the fact that the velocity of the particle decreases with x. At x = L, the wave has its ...
... is small, as well, due to the fact that the probability of observing this fast particle at x ≈ 0 is small. As x becomes larger the wavelength and the amplitude of the particle’s wave gets larger, as well, due to the fact that the velocity of the particle decreases with x. At x = L, the wave has its ...