About the Guide - American Chemical Society
... constricted pipe. Ideal means no boundary layer losses. As the flow moves through the constriction, the pressure, temperature and velocity change, but these variables return to their original values downstream of the constriction. The state of the gas returns to its original conditions and the chang ...
... constricted pipe. Ideal means no boundary layer losses. As the flow moves through the constriction, the pressure, temperature and velocity change, but these variables return to their original values downstream of the constriction. The state of the gas returns to its original conditions and the chang ...
here
... 15. Using the figure above, determine which value equals the latent heat required to change the liquid water into steam. ...
... 15. Using the figure above, determine which value equals the latent heat required to change the liquid water into steam. ...
Cp physics - Fall final review (part II)
... a. the product of the mass of the object and the time interval. b. the product of the force applied to the object and the time interval. c. the time interval divided by the net external force. d. the net external force divided by the time interval. 32. A 0.2 kg baseball is pitched with a velocity of ...
... a. the product of the mass of the object and the time interval. b. the product of the force applied to the object and the time interval. c. the time interval divided by the net external force. d. the net external force divided by the time interval. 32. A 0.2 kg baseball is pitched with a velocity of ...
5.27 MB - KFUPM Resources v3
... 7.5a Reference states and state properties • State property – a property of a system component whose value depends only on the state of the system (i.e. temperature, pressure, phase and composition)… e.g. internal energy (U) and hence, enthalpy (H) • It is impossible to measure the absolute value o ...
... 7.5a Reference states and state properties • State property – a property of a system component whose value depends only on the state of the system (i.e. temperature, pressure, phase and composition)… e.g. internal energy (U) and hence, enthalpy (H) • It is impossible to measure the absolute value o ...
ppt - GeDet
... They are operated close to liquid nitrogen temperature. The mobility of the charge carriers is temperature dependent, and thus so is the rise time of the pulse induced by their drift. Therefore, pulse shape analysis must take into account possible temperature variations. Measurements of the temperat ...
... They are operated close to liquid nitrogen temperature. The mobility of the charge carriers is temperature dependent, and thus so is the rise time of the pulse induced by their drift. Therefore, pulse shape analysis must take into account possible temperature variations. Measurements of the temperat ...
D1 - Status report on the investigation of doping effect on
... Pairs of silicon disks have been successfully bonded in Glasgow using different volumes of sodium silicate bonding solution (1 part commercial sodium silicate solution to 6 parts water, using volumes in the range 0.4l/cm2 to 0.1l/cm2). A complete set of these samples has been sent to Florence for ...
... Pairs of silicon disks have been successfully bonded in Glasgow using different volumes of sodium silicate bonding solution (1 part commercial sodium silicate solution to 6 parts water, using volumes in the range 0.4l/cm2 to 0.1l/cm2). A complete set of these samples has been sent to Florence for ...
Lecture25-12
... We will assume that all processes we discuss are “quasi-static” – they are slow enough that the system is always “in equilibrium” (fluid volumes have the same temperature throughout, etc.) We also assume they are reversible (frictionless pistons, etc.): For a process to be reversible, it must be pos ...
... We will assume that all processes we discuss are “quasi-static” – they are slow enough that the system is always “in equilibrium” (fluid volumes have the same temperature throughout, etc.) We also assume they are reversible (frictionless pistons, etc.): For a process to be reversible, it must be pos ...
File - Ms. Renfro`s Physical Science Web Class
... a. Explain energy transformation in terms of the Law of Conservation of Energy. b. Explain the relationship between potential and kinetic energy. c. Compare and contrast the different forms of energy (heat, light, electricity, mechanical motion, and sound) and their characteristics. d. Describe how ...
... a. Explain energy transformation in terms of the Law of Conservation of Energy. b. Explain the relationship between potential and kinetic energy. c. Compare and contrast the different forms of energy (heat, light, electricity, mechanical motion, and sound) and their characteristics. d. Describe how ...
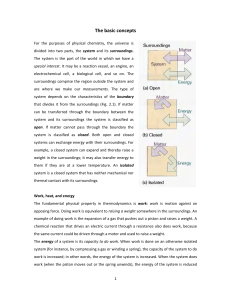
The basic concepts For the purposes of physical chemistry, the
... Suppose a gas is confined by a piston and that the external pressure, Pex is set equal to the pressure, P, of the confined gas. Such a system is in mechanical equilibrium with its surroundings (as illustrated in Section 1.1) because an infinitesimal change in the external pressure in either directio ...
... Suppose a gas is confined by a piston and that the external pressure, Pex is set equal to the pressure, P, of the confined gas. Such a system is in mechanical equilibrium with its surroundings (as illustrated in Section 1.1) because an infinitesimal change in the external pressure in either directio ...
Physical Science Notes ppt.SBP1
... different forms of energy (heat, light, electricity, mechanical motion, sound) and their characteristics. • Energy appears in different forms. Heat energy is in the disorderly motion of molecules (form of thermal) between two objects with different temperatures. Mechanical energy is in moving bodies ...
... different forms of energy (heat, light, electricity, mechanical motion, sound) and their characteristics. • Energy appears in different forms. Heat energy is in the disorderly motion of molecules (form of thermal) between two objects with different temperatures. Mechanical energy is in moving bodies ...
Table of Content
... which depends on the size (i.e., mass) of a system is an extensive property. The total volume of a system is an example of an extensive property. On the other hand, the properties which are independent of the size of a system are called intensive properties. Examples of intensive properties are pres ...
... which depends on the size (i.e., mass) of a system is an extensive property. The total volume of a system is an example of an extensive property. On the other hand, the properties which are independent of the size of a system are called intensive properties. Examples of intensive properties are pres ...
Nano Mechanics and Materials: Theory, Multiscale Methods
... The temperature is introduced as a parameter, which: 1) serves as an intrinsic characteristic of any equilibrium system (similar to V and P) 2) determines thermodynamic equilibrium between two systems in thermal contact Thus, it is postulated that: If two adiabatically isolated systems in equilibriu ...
... The temperature is introduced as a parameter, which: 1) serves as an intrinsic characteristic of any equilibrium system (similar to V and P) 2) determines thermodynamic equilibrium between two systems in thermal contact Thus, it is postulated that: If two adiabatically isolated systems in equilibriu ...
Heat Capacity - Uplift North Hills Prep
... Thermal energy is a term often confused with that of heat. Simply put, heat is the flow of thermal energy. Thermal energy is the total internal energy of the system. This has to do with the kinetic and potential energies of the molecules, i.e. how fast the molecules are vibrating and their chemical ...
... Thermal energy is a term often confused with that of heat. Simply put, heat is the flow of thermal energy. Thermal energy is the total internal energy of the system. This has to do with the kinetic and potential energies of the molecules, i.e. how fast the molecules are vibrating and their chemical ...
LECTURE 26: Work- Kinetic Energy
... This interpretation of the work-energy theorem states that "take the initial kinetic energy of the system and add it to the net external work being done on the system, this addition is equal to the final kinetic energy of the system". It is very important to note, that energy and work are both scala ...
... This interpretation of the work-energy theorem states that "take the initial kinetic energy of the system and add it to the net external work being done on the system, this addition is equal to the final kinetic energy of the system". It is very important to note, that energy and work are both scala ...
energy
... Static form of Energy: total energy of a system can be contained or stored in a system. Dynamic form of Energy: energy interactions, energy in transit; defined at system boundary, represent the energy gained or lost by a system during a process Heat : energy interaction driving by temperature diffe ...
... Static form of Energy: total energy of a system can be contained or stored in a system. Dynamic form of Energy: energy interactions, energy in transit; defined at system boundary, represent the energy gained or lost by a system during a process Heat : energy interaction driving by temperature diffe ...
Chapter 1 - U.S. Coast Guard Auxiliary
... It is a thousand times as large as the calorie. The calorie is defined by the Specific Heat of water—the amount of energy it takes to raise one gram of water one °C. ...
... It is a thousand times as large as the calorie. The calorie is defined by the Specific Heat of water—the amount of energy it takes to raise one gram of water one °C. ...
Lecture Notes 1. Introduction File
... their own such as rotational or vibrational energy whereas molecules will generally have internal motions, available to take up some energy, often in a profusion of different types. I will from now on refer to molecules the term being implicitly understood to cover atoms as well. The system will be ...
... their own such as rotational or vibrational energy whereas molecules will generally have internal motions, available to take up some energy, often in a profusion of different types. I will from now on refer to molecules the term being implicitly understood to cover atoms as well. The system will be ...
Heat of Sublimation - Chemwiki
... for substances in the solid and liquid states. Note that ΔEthermal is divided between ΔPE and ΔKE for substances in the solid and liquid states. This is because the intermolecular and intramolecular forces that exist between the atoms of the substance (i.e. atomic bond, van der Waals forces, etc) ha ...
... for substances in the solid and liquid states. Note that ΔEthermal is divided between ΔPE and ΔKE for substances in the solid and liquid states. This is because the intermolecular and intramolecular forces that exist between the atoms of the substance (i.e. atomic bond, van der Waals forces, etc) ha ...