Week 1: Descriptive Statistics
... numerical value or the “chance” of occurrence of several possible outcomes of an unpredictable event. Engineers study probability because uncertainties are unavoidable in the design and planning of engineering systems ...
... numerical value or the “chance” of occurrence of several possible outcomes of an unpredictable event. Engineers study probability because uncertainties are unavoidable in the design and planning of engineering systems ...
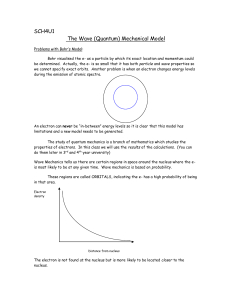
1.1 What has to be explained by Quantum mechanics?
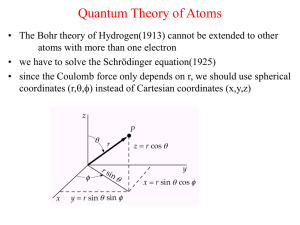
... Only reasonable for Fermions following the Pauli principle! But ”free” and ”occupied” states within a band, sizes of band gaps, etc. classify metals, semiconductors, and insulators. • Why, in contrast, must photons be Bosons?!? (One single QM state macroscopically measurable) • What is: Schrödinger ...
... Only reasonable for Fermions following the Pauli principle! But ”free” and ”occupied” states within a band, sizes of band gaps, etc. classify metals, semiconductors, and insulators. • Why, in contrast, must photons be Bosons?!? (One single QM state macroscopically measurable) • What is: Schrödinger ...
NAME - Electrical and Computer Engineering
... (Gaussian) density with mean 100 and variance 4. (a) Find the probability that X is less than or equal to 105 Ohms. (b) Now lets consider a second factory which also produces 100 Ohm resistors. Again, due to imperfections in the production, the resistors are not exactly 100 Ohms, but have to be trea ...
... (Gaussian) density with mean 100 and variance 4. (a) Find the probability that X is less than or equal to 105 Ohms. (b) Now lets consider a second factory which also produces 100 Ohm resistors. Again, due to imperfections in the production, the resistors are not exactly 100 Ohms, but have to be trea ...
powerpoint
... Superposition creates regions of constructive and destructive diffraction according to the relative incidence of the waves. The light intensity is distributed by the square of the wave envelope: ...
... Superposition creates regions of constructive and destructive diffraction according to the relative incidence of the waves. The light intensity is distributed by the square of the wave envelope: ...
Mock Exam #2 I. DEFINITIONS (10 pts) Define and/or Discuss. You
... 4. ____If P(E) = P(F) then the events are mutually exclusive. 5. ____The following are ALL examples of probability: 1.0, 0.2, 0.0002, and 0.9. 6. By definition, 0! = ____ 7. ____ r = +0.23 indicates a stronger linear relationship than r = -0.62. 8. ____If P(A) = 0.2 and the P(B)=.3, then the P(AorB) ...
... 4. ____If P(E) = P(F) then the events are mutually exclusive. 5. ____The following are ALL examples of probability: 1.0, 0.2, 0.0002, and 0.9. 6. By definition, 0! = ____ 7. ____ r = +0.23 indicates a stronger linear relationship than r = -0.62. 8. ____If P(A) = 0.2 and the P(B)=.3, then the P(AorB) ...
ppt - HEP Educational Outreach
... corresponding to the photon going through either the top or bottom slit. ...
... corresponding to the photon going through either the top or bottom slit. ...
Class23
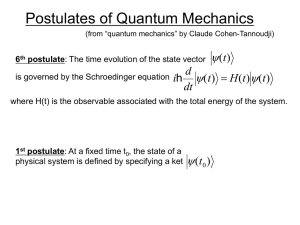
... Quantum mechanics challenges our physical intuition but it is the way things really work. Particles are described with a wave function Y(x,t) which describes the propagation through space and time (when unobserved). ...
... Quantum mechanics challenges our physical intuition but it is the way things really work. Particles are described with a wave function Y(x,t) which describes the propagation through space and time (when unobserved). ...
ST5214 Advanced Probability Theory (Sem 1:2009/10) References
... Probability: Theory and Examples by Durrett, Duxbury Press (main text) Convergence of Stochastic Processes by Pollard, Springer An Introduction to Probability Theory and its Applications by Feller, Wiley Probability and Measure by Billingsley, Wiley ...
... Probability: Theory and Examples by Durrett, Duxbury Press (main text) Convergence of Stochastic Processes by Pollard, Springer An Introduction to Probability Theory and its Applications by Feller, Wiley Probability and Measure by Billingsley, Wiley ...
Probability amplitude

In quantum mechanics, a probability amplitude is a complex number used in describing the behaviour of systems. The modulus squared of this quantity represents a probability or probability density.Probability amplitudes provide a relationship between the wave function (or, more generally, of a quantum state vector) of a system and the results of observations of that system, a link first proposed by Max Born. Interpretation of values of a wave function as the probability amplitude is a pillar of the Copenhagen interpretation of quantum mechanics. In fact, the properties of the space of wave functions were being used to make physical predictions (such as emissions from atoms being at certain discrete energies) before any physical interpretation of a particular function was offered. Born was awarded half of the 1954 Nobel Prize in Physics for this understanding (see #References), and the probability thus calculated is sometimes called the ""Born probability"". These probabilistic concepts, namely the probability density and quantum measurements, were vigorously contested at the time by the original physicists working on the theory, such as Schrödinger and Einstein. It is the source of the mysterious consequences and philosophical difficulties in the interpretations of quantum mechanics—topics that continue to be debated even today.