Unit 2 Student Guided Notes Introduction Carbon is the basic
... acid and an Oxygen further down the chain. An alpha helix contains 3.6 amino acids per spiral. There are other secondary structures, but the alpha helix is the most common and the one you will need to know for this course. Protein Structure - Tertiary and Quaternary Structures The third level is des ...
... acid and an Oxygen further down the chain. An alpha helix contains 3.6 amino acids per spiral. There are other secondary structures, but the alpha helix is the most common and the one you will need to know for this course. Protein Structure - Tertiary and Quaternary Structures The third level is des ...
Arg305 of Streptomyces l-glutamate oxidase plays a crucial role for
... of LGOX resembles that of LAO [19]. A previous report by Pawelek et al. of the crystal structure of LAO interacted with o-aminobenzoate (AB) described that three AB molecules are visible within the funnel of the LAO-AB complex [23]. Likewise, the structure of LGOX interacted with ligand provide us w ...
... of LGOX resembles that of LAO [19]. A previous report by Pawelek et al. of the crystal structure of LAO interacted with o-aminobenzoate (AB) described that three AB molecules are visible within the funnel of the LAO-AB complex [23]. Likewise, the structure of LGOX interacted with ligand provide us w ...
The Pepsin Story - Penn Arts and Sciences
... Have you ever given any thought to how all of the food you eat is converted to energy for your body? In order to use the energy from the food, your body must break down the chemicals into small subunits. One of the digestive processes involves the breaking down of larger polypeptides into smaller. T ...
... Have you ever given any thought to how all of the food you eat is converted to energy for your body? In order to use the energy from the food, your body must break down the chemicals into small subunits. One of the digestive processes involves the breaking down of larger polypeptides into smaller. T ...
Essential Concept of Metabolism
... Enzymes are proteins that catalyze chemical reactions in living organisms by lowering the activation energy needed for a reaction to occur. Enzymes have an active site, the binding site to which the substrate (the substance on which the enzymes act) attaches to form an enzyme-substrate complex. Enzy ...
... Enzymes are proteins that catalyze chemical reactions in living organisms by lowering the activation energy needed for a reaction to occur. Enzymes have an active site, the binding site to which the substrate (the substance on which the enzymes act) attaches to form an enzyme-substrate complex. Enzy ...
Black-Chapter 5 – Essential Concept of Metabolism
... Enzymes are proteins that catalyze chemical reactions in living organisms by lowering the activation energy needed for a reaction to occur. Enzymes have an active site, the binding site to which the substrate (the substance on which the enzymes act) attaches to form an enzyme-substrate complex. Enzy ...
... Enzymes are proteins that catalyze chemical reactions in living organisms by lowering the activation energy needed for a reaction to occur. Enzymes have an active site, the binding site to which the substrate (the substance on which the enzymes act) attaches to form an enzyme-substrate complex. Enzy ...
Biology 231
... adenine (A) – DNA and RNA guanine (G) – DNA and RNA cytosine (C) – DNA and RNA thymine (T) – DNA only uracil (U) – RNA only nucleic acid strands have backbones of sugars and phosphates joined by dehydration reactions RNA is single-stranded DNA is a double-stranded helix held together by hydrogen bon ...
... adenine (A) – DNA and RNA guanine (G) – DNA and RNA cytosine (C) – DNA and RNA thymine (T) – DNA only uracil (U) – RNA only nucleic acid strands have backbones of sugars and phosphates joined by dehydration reactions RNA is single-stranded DNA is a double-stranded helix held together by hydrogen bon ...
Enzyme screening of dikaryotic cultures from lignocellulolitic
... Simultaneous production of laccase and MnP was observed for all cultures; demonstrating that these fungi could be useful for studying co-operation between oxidases and peroxidases during lignin degradation. In this work, although no additional chemicals were used to stimulate enzymatic production, l ...
... Simultaneous production of laccase and MnP was observed for all cultures; demonstrating that these fungi could be useful for studying co-operation between oxidases and peroxidases during lignin degradation. In this work, although no additional chemicals were used to stimulate enzymatic production, l ...
SELECTIVE INHIBITORS OF DIHYDROFOLATE REDUCTASE
... number of identities. If one takes into account enzymes not in the mainstream, e.g. those from protozoa plasmids, the number is even smaller. The mainstream enzymes (from bacterial and mammalian sources) have similar conformations. That published by Richardson (16) may be regarded as the type (Fig. ...
... number of identities. If one takes into account enzymes not in the mainstream, e.g. those from protozoa plasmids, the number is even smaller. The mainstream enzymes (from bacterial and mammalian sources) have similar conformations. That published by Richardson (16) may be regarded as the type (Fig. ...
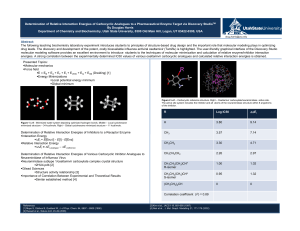
Determination of Relative Interaction Energies of Carbocyclic
... Determination of Relative Interaction Energies of Carbocyclic Analogues to a Pharmaceutical Enzyme Target via Discovery StudioTM By Douglas Harris Department of Chemistry and Biochemistry, Utah State University, 0300 Old Main Hill, Logan, UT 83422-0300, USA [email protected] ...
... Determination of Relative Interaction Energies of Carbocyclic Analogues to a Pharmaceutical Enzyme Target via Discovery StudioTM By Douglas Harris Department of Chemistry and Biochemistry, Utah State University, 0300 Old Main Hill, Logan, UT 83422-0300, USA [email protected] ...
Ch. 2 Notes Organic Chemistry
... Make chemical rxns happen in living organisms. Example: salivary amylase in saliva– begins process of digestion ...
... Make chemical rxns happen in living organisms. Example: salivary amylase in saliva– begins process of digestion ...
Metabolism of Members of the Spiroplasmataceae
... discussed previously, problems associated with studies in which researchers use crude cell extracts from organisms grown in rich undefined media may lead to incorrect conclusions concerning the presence or absence of enzymatic activity (11, 28). Such errors may be due to low assay sensitivity, conta ...
... discussed previously, problems associated with studies in which researchers use crude cell extracts from organisms grown in rich undefined media may lead to incorrect conclusions concerning the presence or absence of enzymatic activity (11, 28). Such errors may be due to low assay sensitivity, conta ...
m5zn_c04497c09413e2c
... usually ends in ase identifies the reacting substance; for example, sucrase catalyzes the reaction of sucrose describes the function of the enzyme; for example, oxidases catalyze oxidation can be a common name, particularly for the digestive enzymes (such as pepsin and trypsin) ...
... usually ends in ase identifies the reacting substance; for example, sucrase catalyzes the reaction of sucrose describes the function of the enzyme; for example, oxidases catalyze oxidation can be a common name, particularly for the digestive enzymes (such as pepsin and trypsin) ...
Visualizing Biological Pathways
... for fermentation in 1860. • Eduard Buchner discovered that extracts of certain cells can cause fermentation in 1897. • Arthur Harden and William Young determined that a heatsensitive high-molecular-weight subcellular fraction (the enzymes) and a heat-insensitive low-molecular-weight cytoplasm fracti ...
... for fermentation in 1860. • Eduard Buchner discovered that extracts of certain cells can cause fermentation in 1897. • Arthur Harden and William Young determined that a heatsensitive high-molecular-weight subcellular fraction (the enzymes) and a heat-insensitive low-molecular-weight cytoplasm fracti ...
LE - 2 - Organic Molecules
... • How does the food turn into molecules that run and build our body? • The food gets broken down into simple units. Then those units are used or assembled in our bodies and turn into something we need! ...
... • How does the food turn into molecules that run and build our body? • The food gets broken down into simple units. Then those units are used or assembled in our bodies and turn into something we need! ...
copyrighted material
... homeostasis is maintained by negative feedback. After a meal, the absorption of glucose (a sugar) from the digestive tract increases the amount of glucose in the blood. In response, specialized cells in the pancreas (alpha cells) secrete the hormone insulin, which circulates through the blood and st ...
... homeostasis is maintained by negative feedback. After a meal, the absorption of glucose (a sugar) from the digestive tract increases the amount of glucose in the blood. In response, specialized cells in the pancreas (alpha cells) secrete the hormone insulin, which circulates through the blood and st ...
magnesium chloride TDS
... EcoR I and EcoR V, and Ribonuclease H. Magnesium also stabilizes polymeric nucleic acids ...
... EcoR I and EcoR V, and Ribonuclease H. Magnesium also stabilizes polymeric nucleic acids ...
History and Function
... Therefore, RNase A is referred to RNA depolymerase The imidazole group of His12 acts as a base in the transphosphorylation reaction and an acid in the hydrolysis reaction The imidazole group of His 119 has complementary role, acting as an acid in the trasphosphorylation reaction and a base in the hy ...
... Therefore, RNase A is referred to RNA depolymerase The imidazole group of His12 acts as a base in the transphosphorylation reaction and an acid in the hydrolysis reaction The imidazole group of His 119 has complementary role, acting as an acid in the trasphosphorylation reaction and a base in the hy ...
Reassembled Biosynthetic Pathway for Large
... nomically feasible. Without extensive optimization, the current system produces 3 ± 4 g of Gala1,3Lac trisaccharide from every 10 L fermentation. Based on the commercial prices for the chemical reagents and growth medium used, the material cost for the production is about $ 25 per gram of product. L ...
... nomically feasible. Without extensive optimization, the current system produces 3 ± 4 g of Gala1,3Lac trisaccharide from every 10 L fermentation. Based on the commercial prices for the chemical reagents and growth medium used, the material cost for the production is about $ 25 per gram of product. L ...
Document
... They are the principal form of stored energy in most organisms & major constituents of cellular membranes. Specialised lipids serve as pigments ( Retinol, carotene); Cofactors ( vitamin K); Detergents ( bile salts) etc. Anchors for membrane proteins ( Signalling) ...
... They are the principal form of stored energy in most organisms & major constituents of cellular membranes. Specialised lipids serve as pigments ( Retinol, carotene); Cofactors ( vitamin K); Detergents ( bile salts) etc. Anchors for membrane proteins ( Signalling) ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.