3-30 An exposed hot surface of an industrial natural gas furnace is
... that section of the wall by 90 percent. The thickness of the insulation that needs to be used is to be determined. Also, the length of time it will take for the insulation to pay for itself from the energy it saves will be determined. Assumptions 1 Heat transfer through the wall is steady and one-di ...
... that section of the wall by 90 percent. The thickness of the insulation that needs to be used is to be determined. Also, the length of time it will take for the insulation to pay for itself from the energy it saves will be determined. Assumptions 1 Heat transfer through the wall is steady and one-di ...
EQ: How can heat be transferred from one place to another?
... temperature if not eaten quickly. BUT!!! Did the food cool or did the surrounding air warm? Why? In science, there is no such thing as coldness. Instead, the matter grows colder as thermal energy flows from it and transfers to another object/form of matter. Heat transfer occurs only in one direction ...
... temperature if not eaten quickly. BUT!!! Did the food cool or did the surrounding air warm? Why? In science, there is no such thing as coldness. Instead, the matter grows colder as thermal energy flows from it and transfers to another object/form of matter. Heat transfer occurs only in one direction ...
module 7
... Second Law considerations but, for the undergraduate student, it is generally more satisfying to arbitrarily choose a set of temperatures and check the results from the two equations. The only restrictions that we place on the case is that it be physically possible for parallel flow, i.e. 1 and 2 ...
... Second Law considerations but, for the undergraduate student, it is generally more satisfying to arbitrarily choose a set of temperatures and check the results from the two equations. The only restrictions that we place on the case is that it be physically possible for parallel flow, i.e. 1 and 2 ...
Thermodynamics
... specific heat--the quantity of heat energy needed to raise a specific amount of a substance by one degree. 4.186 J/ gC °C for water. Water’s specific heat is unusually high due to ...
... specific heat--the quantity of heat energy needed to raise a specific amount of a substance by one degree. 4.186 J/ gC °C for water. Water’s specific heat is unusually high due to ...
APPLICATIONS OF MICROCALORIMETRY IN STABILITY STUDIES INTRODUCTION:
... A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction. Bomb calorimeters have to withstand the large pressure within the calorimeter as the reaction is being measured. Electrical energy is used to ignite the fuel; as the fuel is ...
... A bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of a particular reaction. Bomb calorimeters have to withstand the large pressure within the calorimeter as the reaction is being measured. Electrical energy is used to ignite the fuel; as the fuel is ...
Thermodynamics - Faculty
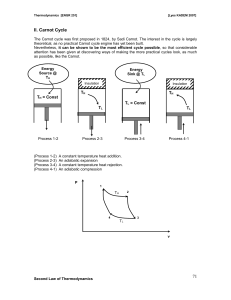
... a) Step 1: The cycle starts with the piston positioned such that V is at a minimum. At this point, heat Q is added to the system through a heat reservoir at a high temperature TH . The system absorbs the heat a constant temperature TH which causes the volume to expand doing work on the piston. Durin ...
... a) Step 1: The cycle starts with the piston positioned such that V is at a minimum. At this point, heat Q is added to the system through a heat reservoir at a high temperature TH . The system absorbs the heat a constant temperature TH which causes the volume to expand doing work on the piston. Durin ...
Anonymous-IntroductiontoThermodynamics-qsp_chapte+
... besides molecular motions - the molecules may vibrate and rotate, the material may be placed in an electric or magnetic field, the energy density of radiation may be important and so on. Therefore, we need a general way of coping with all these possibilities. In general, there are two ways in which ...
... besides molecular motions - the molecules may vibrate and rotate, the material may be placed in an electric or magnetic field, the energy density of radiation may be important and so on. Therefore, we need a general way of coping with all these possibilities. In general, there are two ways in which ...
PX121: Thermal Physics Lecture 2
... “A system in equilibrium reacts to a (small) externally imposed change in one of its state variables by readjusting its internal condition so as to reverse the change.” (Henri Louis le Chatelier, 1844) E.g. ...
... “A system in equilibrium reacts to a (small) externally imposed change in one of its state variables by readjusting its internal condition so as to reverse the change.” (Henri Louis le Chatelier, 1844) E.g. ...
Calorimetry – Heats of Solution
... where q is the heat flow (in calories), S.H. stands for the specific heat of the reaction solution (in cal/g·oC), m is the mass of the solution (in g), and ΔT is the change in temperature (in oC). For an exothermic reaction, qreaction is negative. The heat released by the reaction will increase the ...
... where q is the heat flow (in calories), S.H. stands for the specific heat of the reaction solution (in cal/g·oC), m is the mass of the solution (in g), and ΔT is the change in temperature (in oC). For an exothermic reaction, qreaction is negative. The heat released by the reaction will increase the ...
Thermodynamics: Notes
... Some processes, we see, will involve heat flow. In these processes, we want the system to be in equilibrium. So we require a quasistatic process. Thus, every process involving heat flow is to be quasistatic. Further, we have banned dissipative forces from the system. Therefore, these processes are r ...
... Some processes, we see, will involve heat flow. In these processes, we want the system to be in equilibrium. So we require a quasistatic process. Thus, every process involving heat flow is to be quasistatic. Further, we have banned dissipative forces from the system. Therefore, these processes are r ...
8. Temperature and Heat - City, University of London
... It takes 4186J of heat to raise the temperature of 1kg of water by 1°C The heat required for a 1°C increase varies from one substance to another, e.g. it takes only 129J of heat to increase the temperature of lead by 1°C The heat required for a given increase in temperature is given by the heat capa ...
... It takes 4186J of heat to raise the temperature of 1kg of water by 1°C The heat required for a 1°C increase varies from one substance to another, e.g. it takes only 129J of heat to increase the temperature of lead by 1°C The heat required for a given increase in temperature is given by the heat capa ...
Finite-time thermodynamic analysis of an irreversible vacuum
... The VTPG solar cell composed of a concentrator, a collector, a heat sink and VTPGS connected in series exchange heat with the collector and heat sink, as shown in Fig. 1 (a). The collector acts as the high-temperature heat reservoir of the VTPG solar cell for a further production of electric energy. ...
... The VTPG solar cell composed of a concentrator, a collector, a heat sink and VTPGS connected in series exchange heat with the collector and heat sink, as shown in Fig. 1 (a). The collector acts as the high-temperature heat reservoir of the VTPG solar cell for a further production of electric energy. ...
physical chemistry lecture 3
... •Some examples include energy (and many other thermodynamic terms), pressure, volume, altitude, distance, etc. • An energy change in a system can occur by many different combinations of heat (q) and work (w), but no matter what the combination, ΔU is always the same — the amount of the energy change ...
... •Some examples include energy (and many other thermodynamic terms), pressure, volume, altitude, distance, etc. • An energy change in a system can occur by many different combinations of heat (q) and work (w), but no matter what the combination, ΔU is always the same — the amount of the energy change ...
Heat equation

The heat equation is a parabolic partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time.