* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 16-Chapter 7-October 19, 2005
Survey
Document related concepts
Transcript
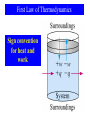
General Chemistry Principles and Modern Applications Petrucci • Harwood • Herring 8th Edition Chapter 7: Thermochemistry Philip Dutton University of Windsor, Canada N9B 3P4 Prentice-Hall © 2002 (modified 2003 by Dr. Paul Root and 2005 by Dr. David Tramontozzi) Contents 7-1 7-2 7-3 7-4 7-5 7-6 7-7 Getting Started: Some Terminology Heat Heats of Reaction and Calorimetry Work The First Law of Thermodynamics Heats of Reaction: U and H The Indirect Determination of H: Hess’s Law Contents 7-7 The Indirect Determination of H, Hess’s Law 7-8 Standard Enthalpies of Formation 7-9 Fuels as Sources of Energy Focus on Fats, Carbohydrates, and Energy Storage. Things to Remember q system + q surroundings = 0 q = mcT •Heat of reaction, qrxn. –The quantity of heat exchanged between a system and its surroundings when a chemical reaction occurs within the system, at constant temperature. Coffee Cup Calorimeter • A simple calorimeter. – Well insulated and therefore isolated. – Measure temperature change. qrxn = -qcal Example 7-4 Two solutions, 100 mL of 1.00 M HCl(aq) and 100 mL of 1.00 M NaOH(aq), both initially at 21.1 ˚C, are added to a styrofoam cup calorimeter and allowed to react. The temperature rises to 27.8 ˚C. Determine the heat of the neutralization reaction, expressed per mole of H2O formed. Is the reaction endothermic or exothermic? H+(aq) + OH-(aq) H2O(l) Example 7-4 qreaction = - qcalorimeter Given : qcalorimeter = mcΔT m = 200 g of H2O c = 4.18 J g-1˚C-1 ΔT = (27.8˚C - 21.1˚C) = 6.7 ˚C qcalorimeter = mcΔT = (200 g)(4.18 J g-1˚C-1)(6.7 ˚C) = 5600 J Example 7-4 qreaction = - qcalorimeter qreaction = -5600 J mols of H+ = 0.100 L x 1.0 mol/L = 0.1 mol H+ mols of H2O = 0.1 mol H+ x 1.0 mol H20 / 1.0 mol H+ = 0.1 mol H2O Amount of heat produced per mole H2O qreaction = -56000 J / mole H2O or -56 kJ mol-1 Since qreaction < 0, this reaction is exothermic 7-4 Work • In addition to heat effects chemical reactions may also do work. • Gas formed pushes against the atmosphere. • Volume changes. • Pressure-volume work. Pressure Volume Work w=Fd = (P A) h = PV w = -PextV Example 7-5 7-3 Calculating Pressure-Volume Work. Suppose the gas in the previous figure is 0.100 mol He at 298 K. How much work, in Joules, is associated with its expansion at constant pressure. Assume an ideal gas and calculate the volume change: Vi = nRT/P = (0.100 mol)(0.08201 L atm mol-1 K-1)(298K)/(2.40 atm) = 1.02 L Vf = 1.88 L V = 1.88-1.02 L = 0.86 L Example 7-5 7-3 Calculate the work done by the system: w = -PV = -(1.30 atm)(0.86 L)( = -1.1 102 J -101 J ) 1 L atm Hint: If you use pressure in kPa you get Joules directly. Where did the conversion factor come from? Compare two versions of the gas constant and calculate. 8.3145 J/mol K ≡ 0.082057 L atm/mol K 1 ≡ 101.33 J/L atm 7-5 The First Law of Thermodynamics • Internal Energy, U. – Total energy (potential and kinetic) in a system. •Translational kinetic energy. •Molecular rotation. •Bond vibration. •Intermolecular attractions. •Chemical bonds. •Electrons. First Law of Thermodynamics • A system contains only internal energy. – A system does not contain heat or work. – These only occur during a change in the system. U = q + w • Law of Conservation of Energy – The energy of an isolated system is constant First Law of Thermodynamics Sign convention for heat and work State Functions • Any property that has a unique value for a specified state of a system is said to be a State Function. • Water at 293.15 K and 1.00 atm is in a specified state. • d = 0.99820 g/mL • This density is a unique function of the state. • It does not matter how the state was established. Functions of State • U is a function of state. – Not easily measured. • U has a unique value between two states. – Is easily measured. Path Dependent Functions • Changes in heat and work are not functions of state. – Remember example 7-5, w = -1.1 102 J in a one step expansion of gas – Consider 2.40 atm to 1.80 atm and finally to 1.30 atm. w = (-1.80 atm)(1.30-1.02)L – (1.30 atm)(1.88-1.36)L = -0.61 L atm – 0.68 L atm = -1.3 L atm = -1.3 102 J 7-6 Heats of Reaction: U and H Reactants → Products Ui Uf U = Uf - Ui U = qrxn + w In a system at constant volume: U = qrxn + 0 = qrxn = qv But we live in a constant pressure world! How does qp relate to qv? Heats of Reaction Burn gasoline in a bomb calorimeter Burn gasoline in an automobile engine Heats of Reaction qV = qP + w We know that w = - PV and U = qV, therefore: U = qP - PV qP = U + PV These are all state functions, so define a new function. Let H = U + PV Then H = Hf – Hi = U + PV If we work at constant pressure and temperature: H = U + PV = qP Comparing Heats of Reaction qP = -566 kJ/mol = H PV = P(Vf – Vi) = RT(nf – ni) = -2.5 kJ U = H - PV = -563.5 kJ/mol = qV Changes of State of Matter Molar enthalpy of vaporization: H2O (l) → H2O(g) H = 44.0 kJ at 298 K Molar enthalpy of fusion: H2O (s) → H2O(l) H = 6.01 kJ at 273.15 K Example 7-8 7-3 Enthalpy Changes Accompanying Changes in States of Matter. Calculate H for the process in which 50.0 g of water is converted from liquid at 10.0°C to vapor at 25.0°C. Break the problem into two steps: Raise the temperature of the liquid first then completely vaporize it. The total enthalpy change is the sum of the changes in each step. Set up the equation and calculate: qP = mcH2OT + nHvap 50.0 g = (50.0 g)(4.184 J/g °C)(25.0-10.0)°C + 44.0 kJ/mol 18.0 g/mol = 3.14 kJ + 122 kJ = 125 kJ






























![Second review [Compatibility Mode]](http://s1.studyres.com/store/data/003692853_1-a578e4717b0c8365c11d7e7f576654ae-150x150.png)



