Equivalence of Kelvin-Planck and Clausius statements
... that negligible heat transfer takes place between the gas and the surroundings during this process. At the end of the unrestrained expansion process, the gas (system) has the same internal energy, as it had initially. ...
... that negligible heat transfer takes place between the gas and the surroundings during this process. At the end of the unrestrained expansion process, the gas (system) has the same internal energy, as it had initially. ...
More Thermodynamics
... (6) therefore states that the internal energy of a system expanded at constant temperature will change, and this change is due to attractive interactions between molecules. Since the ideal gas equation of state neglects these interactions, it predicts no change in the internal energy upon expansion ...
... (6) therefore states that the internal energy of a system expanded at constant temperature will change, and this change is due to attractive interactions between molecules. Since the ideal gas equation of state neglects these interactions, it predicts no change in the internal energy upon expansion ...
Chemistry Goal 2 Study Guide
... i. How many moles of sulfur must be burned to give 0.567 moles of SO2? 0.567 mol ii. How many moles of SO2 can be produced from 67.1 moles of O2? 67.1 mol c. Balance the chemical equation and use it to solve for the following problems: i. Al(s) + H2SO4(aq) →AlSO4 + H2 1. If 45.60 L of Hydrogen gas f ...
... i. How many moles of sulfur must be burned to give 0.567 moles of SO2? 0.567 mol ii. How many moles of SO2 can be produced from 67.1 moles of O2? 67.1 mol c. Balance the chemical equation and use it to solve for the following problems: i. Al(s) + H2SO4(aq) →AlSO4 + H2 1. If 45.60 L of Hydrogen gas f ...
17.1
... b) Heat always flows from warmer objects to cooler objects. c) Adding heat can cause an increase in the temperature of an object. d) Heat cannot be specifically detected by senses or instruments. ...
... b) Heat always flows from warmer objects to cooler objects. c) Adding heat can cause an increase in the temperature of an object. d) Heat cannot be specifically detected by senses or instruments. ...
Thermal mass - City of Hobart
... ‘Thermal mass’ describes materials which have the ability to absorb and store heat. Generally, the heavier and denser the material, the more heat they will store, and the longer it will take to release that heat. To take as full advantage of the sun’s energy as possible, buildings need to be constru ...
... ‘Thermal mass’ describes materials which have the ability to absorb and store heat. Generally, the heavier and denser the material, the more heat they will store, and the longer it will take to release that heat. To take as full advantage of the sun’s energy as possible, buildings need to be constru ...
Investigate the Combustion of Alcohols
... carried out the activity safely, particularly with regard to measuring out and transferring water to the calorimeter and to recording weighings and temperatures to an appropriate precision in addition to safe handling of the spirit burners. Students should not be judged on their ability to calculate ...
... carried out the activity safely, particularly with regard to measuring out and transferring water to the calorimeter and to recording weighings and temperatures to an appropriate precision in addition to safe handling of the spirit burners. Students should not be judged on their ability to calculate ...
A-level Chemistry Task Task: PSA09 - Investigate the
... carried out the activity safely, particularly with regard to measuring out and transferring water to the calorimeter and to recording weighings and temperatures to an appropriate precision in addition to safe handling of the spirit burners. Students should not be judged on their ability to calculate ...
... carried out the activity safely, particularly with regard to measuring out and transferring water to the calorimeter and to recording weighings and temperatures to an appropriate precision in addition to safe handling of the spirit burners. Students should not be judged on their ability to calculate ...
IOSR Journal of Mathematics (IOSR-JM)
... Combined heat transfer processes such as convection-radiation play a significant role in several chemical processes involving combustion, drying, fluidization, MHD flows, and so forth. In general, the radiative process either occurs at the boundaries or as a term in the energy equation. In the latte ...
... Combined heat transfer processes such as convection-radiation play a significant role in several chemical processes involving combustion, drying, fluidization, MHD flows, and so forth. In general, the radiative process either occurs at the boundaries or as a term in the energy equation. In the latte ...
JIF 314 Thermodynamics - comsics
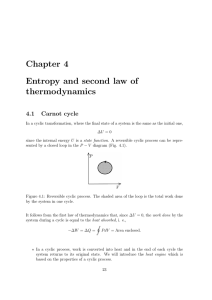
... If initially the pot contains boiling water, the temperature (X) is not constant but will keep dropping. Hence during this period, both system A and B are not in TE Over the time, when X temperature drops to a value equal to X, both X and X will change no more. We say that the water in the pot and ...
... If initially the pot contains boiling water, the temperature (X) is not constant but will keep dropping. Hence during this period, both system A and B are not in TE Over the time, when X temperature drops to a value equal to X, both X and X will change no more. We say that the water in the pot and ...
Read each question. Then fill in the correct answer on the answer
... 16. The volume of a rectangular prism is 864 cubic centimeters. The length is 1 centimeter less than the height, and the width is 3 centimeters more than the height. a. Write a polynomial equation that can be used to solve for the height of the prism h. b. How many possible roots are there for h in ...
... 16. The volume of a rectangular prism is 864 cubic centimeters. The length is 1 centimeter less than the height, and the width is 3 centimeters more than the height. a. Write a polynomial equation that can be used to solve for the height of the prism h. b. How many possible roots are there for h in ...
HEAT
... The energy used or produced in a chemical reaction is called the enthalpy of the reaction •Burning a 15 gram piece of paper produces a particular amount of heat energy or a particular amount of enthalpy •Enthalpy is a value that also contains a component of direction (energy in or energy out) Heat g ...
... The energy used or produced in a chemical reaction is called the enthalpy of the reaction •Burning a 15 gram piece of paper produces a particular amount of heat energy or a particular amount of enthalpy •Enthalpy is a value that also contains a component of direction (energy in or energy out) Heat g ...
Elementary Notes on Classical Thermodynamics
... Let us, now, briefly comment equation (3). That equation is about energy change. We can think of the system as possessing a certain amount of energy due to various processes which are taking place inside it. It is important to understand that in Thermodynamics we are not interested to the energy acq ...
... Let us, now, briefly comment equation (3). That equation is about energy change. We can think of the system as possessing a certain amount of energy due to various processes which are taking place inside it. It is important to understand that in Thermodynamics we are not interested to the energy acq ...
Heat equation

The heat equation is a parabolic partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time.