Le Châtelier`s Principle
... • Make more reactants because more collisions with the products will occur • A stress was applied and the system compensated for this change What will removing CO2 do? • Shift to make more products ...
... • Make more reactants because more collisions with the products will occur • A stress was applied and the system compensated for this change What will removing CO2 do? • Shift to make more products ...
Thermochemistry PPT
... • Thermochemistry – study of energy changes that occur during phase changes and chem. rxns. • Chem. Potential Energy – energy stored in chemical bonds. ...
... • Thermochemistry – study of energy changes that occur during phase changes and chem. rxns. • Chem. Potential Energy – energy stored in chemical bonds. ...
Heat Transfer - Madison County Schools
... of a fluid take heat with them as they move. If you heat the air in one room, the air will heat the next room as the air flows from one room to the next. This is heating by convection. ...
... of a fluid take heat with them as they move. If you heat the air in one room, the air will heat the next room as the air flows from one room to the next. This is heating by convection. ...
Physical Property Notes
... 1) Heat up a known mass of a metal in boiling water until the substance has reached the same temperature as the boiling water (100oC) 2) Transfer (very quickly) the hot metal to a water bath that contains a specific amount of water at a known temperature (usually the temperature of the room) 3) Meas ...
... 1) Heat up a known mass of a metal in boiling water until the substance has reached the same temperature as the boiling water (100oC) 2) Transfer (very quickly) the hot metal to a water bath that contains a specific amount of water at a known temperature (usually the temperature of the room) 3) Meas ...
18493 Demonstrate knowledge of heat transfer in a seafood
... Radiation refers to the transfer of heat energy by electromagnetic waves. Examples of radiation include infra-red cooking; Latent heat refers to the heat required to change, at constant temperature, the physical state of materials from solid to liquid, liquid to gas, and solid to gas; Sensible heat ...
... Radiation refers to the transfer of heat energy by electromagnetic waves. Examples of radiation include infra-red cooking; Latent heat refers to the heat required to change, at constant temperature, the physical state of materials from solid to liquid, liquid to gas, and solid to gas; Sensible heat ...
To Measure the Specific Latent heat of Fusion of Ice
... From this equation you can calculate the specific latent heat of fusion of ice. The accepted value is 334,000 J/kg. Try to explain why your experiment gave a different answer. Were your 2 results very different? Data Collection: Record temperature, mass of water and mass of ice in a suitable table ...
... From this equation you can calculate the specific latent heat of fusion of ice. The accepted value is 334,000 J/kg. Try to explain why your experiment gave a different answer. Were your 2 results very different? Data Collection: Record temperature, mass of water and mass of ice in a suitable table ...
Thermodynamics Guided Notes
... in at the end of the unit completed by its owner. If you are absent any days during this unit, it is your responsibility to get notes from a helpful classmate then check with me to see what you missed in class. It is very important to keep up with the reading and homework from this unit since we don ...
... in at the end of the unit completed by its owner. If you are absent any days during this unit, it is your responsibility to get notes from a helpful classmate then check with me to see what you missed in class. It is very important to keep up with the reading and homework from this unit since we don ...
Thermochemistry
... Measuring Energy Changes • calorie(c)-amount of energy required to raise the temperature of one gram of water by one Celsius degree. (Food you eat is measured in Kilocalories which is abbreviated C). • Joule (J)-the SI unit of energy • 1 c=4.184J ...
... Measuring Energy Changes • calorie(c)-amount of energy required to raise the temperature of one gram of water by one Celsius degree. (Food you eat is measured in Kilocalories which is abbreviated C). • Joule (J)-the SI unit of energy • 1 c=4.184J ...
Specific Heat!
... changes from a temperature of 20.0°C to 27.4ºC. How much heat energy did it gain? A 200 J ...
... changes from a temperature of 20.0°C to 27.4ºC. How much heat energy did it gain? A 200 J ...
thermodynamics, heat and mass transfer
... 1. During the working stroke of an engine the heat transferred out of the system was 150 kJ/kg of working substance. The internal energy also decreased by 400 kJ/kg of working substance. Determine the work done and state whether it is work done on or by the engine. 2. Air in a closed vessel of fixed ...
... 1. During the working stroke of an engine the heat transferred out of the system was 150 kJ/kg of working substance. The internal energy also decreased by 400 kJ/kg of working substance. Determine the work done and state whether it is work done on or by the engine. 2. Air in a closed vessel of fixed ...
Heat Transfer Conduction, Convection, and Radiation
... Examples of Convection: • Have you ever noticed that the air near the ceiling is warmer than the air near the floor? Or that water in a pool is cooler at the deep end? • Examples: air movement in a home, pot of heating water. • Pick one of these examples and draw the circular pattern in your notes. ...
... Examples of Convection: • Have you ever noticed that the air near the ceiling is warmer than the air near the floor? Or that water in a pool is cooler at the deep end? • Examples: air movement in a home, pot of heating water. • Pick one of these examples and draw the circular pattern in your notes. ...
Thermal Energy
... b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C ...
... b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C ...
Thermochemistry www.AssignmentPoint.com Thermochemistry is
... enclosed chamber within which the change to be examined occurs. The temperature of the chamber is monitored either using a thermometer or thermocouple, and the temperature plotted against time to give a graph from which fundamental quantities can be calculated. Modern calorimeters are ...
... enclosed chamber within which the change to be examined occurs. The temperature of the chamber is monitored either using a thermometer or thermocouple, and the temperature plotted against time to give a graph from which fundamental quantities can be calculated. Modern calorimeters are ...
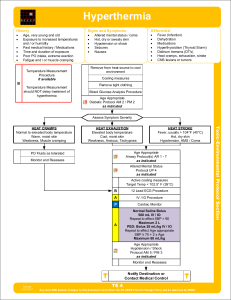
Heat wave

A heat wave is a prolonged period of excessively hot weather, which may be accompanied by high humidity, especially in oceanic climate countries. While definitions vary, a heat wave is measured relative to the usual weather in the area and relative to normal temperatures for the season. Temperatures that people from a hotter climate consider normal can be termed a heat wave in a cooler area if they are outside the normal climate pattern for that area.The term is applied both to routine weather variations and to extraordinary spells of heat which may occur only once a century. Severe heat waves have caused catastrophic crop failures, thousands of deaths from hyperthermia, and widespread power outages due to increased use of air conditioning. A heat wave is considered extreme weather, and a danger because heat and sunlight may overheat the human body.