Internal Energy
... The first law of thermodynamics states that the internal energy of a system is conserved. Q is the heat that is added to the system • If heat is lost, Q is negative. W is the work done by the system. • If work is done on the system, W is negative ...
... The first law of thermodynamics states that the internal energy of a system is conserved. Q is the heat that is added to the system • If heat is lost, Q is negative. W is the work done by the system. • If work is done on the system, W is negative ...
energy
... • Heat is added at a constant rate • When a substance is undergoing a phase change THE TEMPERATURE DOES NOT CHANGE until the phase change is complete ...
... • Heat is added at a constant rate • When a substance is undergoing a phase change THE TEMPERATURE DOES NOT CHANGE until the phase change is complete ...
Thermal Chem Review and Key
... diagonal or plateau sections on your heating and cooling curve? 16. What equation is used to calculate the energy involved in increasing or decreasing the temperature of a substance? Does this equation pertain to the diagonal or plateau sections on your heating and cooling curve? 17. What does it me ...
... diagonal or plateau sections on your heating and cooling curve? 16. What equation is used to calculate the energy involved in increasing or decreasing the temperature of a substance? Does this equation pertain to the diagonal or plateau sections on your heating and cooling curve? 17. What does it me ...
An Approach to a Zero
... temperature exceeds the specified upper bound on thermal comfort And solar gain, heat from occupants, and heat dissipated from lights and appliances only make things worse. But there’s a trick. The occupants can open the windows or doors and hope that wind-driven airflows are sufficient to bring the ...
... temperature exceeds the specified upper bound on thermal comfort And solar gain, heat from occupants, and heat dissipated from lights and appliances only make things worse. But there’s a trick. The occupants can open the windows or doors and hope that wind-driven airflows are sufficient to bring the ...
Thermo Equilibrium Essay - Dyno Guzman`s Digital Portfolio
... The zeroth law of thermodynamics is a generalization about the thermal equilibrium among bodies, or thermodynamic systems. It results from the definition and properties of temperature. ...
... The zeroth law of thermodynamics is a generalization about the thermal equilibrium among bodies, or thermodynamic systems. It results from the definition and properties of temperature. ...
Comparison of Heat Loss by Sample Building Component
... Comparison of Heat Loss by Sample Building Component Windows vs. Walls Formula: Heat Loss (BTU/hr) = UA Where U = Thermal transmittance Where A = Area Where = Delta T (temperature difference) Use the formula for calculating heat loss to discuss window replacement as an energy savings measure. Use th ...
... Comparison of Heat Loss by Sample Building Component Windows vs. Walls Formula: Heat Loss (BTU/hr) = UA Where U = Thermal transmittance Where A = Area Where = Delta T (temperature difference) Use the formula for calculating heat loss to discuss window replacement as an energy savings measure. Use th ...
Science Unit 5 Powerpoint 2 Energy
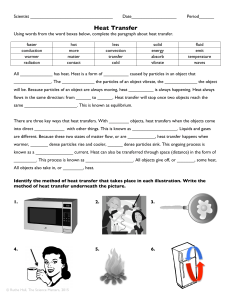
... heat will continue to flow until the temperature of the two objects has equalized, or reached the same temperature. For example, suppose you place an ice cube in a glass of water. Because the water is warmer than the ice, heat flows from the water to the ice until the two reach the same temperature. ...
... heat will continue to flow until the temperature of the two objects has equalized, or reached the same temperature. For example, suppose you place an ice cube in a glass of water. Because the water is warmer than the ice, heat flows from the water to the ice until the two reach the same temperature. ...
doc - University of Colorado Boulder
... from a human being consuming 2,500 kilo-calories (or roughly 10,000,000 Joules per day, assuming the human being is at thermal steady state (doesn’t grow and doesn’t warm up). Q = E / t = ________________________________________ ...
... from a human being consuming 2,500 kilo-calories (or roughly 10,000,000 Joules per day, assuming the human being is at thermal steady state (doesn’t grow and doesn’t warm up). Q = E / t = ________________________________________ ...
Joule`s Law and Heat Transfer Name
... To do this we need to measure the amount of electrical energy we supply and the amount of heat energy the water, calorimeter-cup, and heater gains. Then neglecting heat loss to the room, we could find the relationship between calorie and Joule. Electrical energy, E is given by: E = I V t; where I = ...
... To do this we need to measure the amount of electrical energy we supply and the amount of heat energy the water, calorimeter-cup, and heater gains. Then neglecting heat loss to the room, we could find the relationship between calorie and Joule. Electrical energy, E is given by: E = I V t; where I = ...
LECTURE 5 Temperature Scales The equation of state of any
... Qualitatively one can think of the heat capacity as a measure of the ability of the system to hold heat. The more heat it can hold, the higher its heat capacity. In places of the country where it’s cold in the winter, there is often a radiator in each room which has hot water circulating through it ...
... Qualitatively one can think of the heat capacity as a measure of the ability of the system to hold heat. The more heat it can hold, the higher its heat capacity. In places of the country where it’s cold in the winter, there is often a radiator in each room which has hot water circulating through it ...
Convection Currents and the Mantle
... As you already know, the earth's mantle contains two layers; the stratosphere (tough liquid part of the outer mantle) and the lithosphere (the stiffer outer mantle and the crust). Because of the intense pressure and temperature in the mantle convection currents occur. To learn about what influence t ...
... As you already know, the earth's mantle contains two layers; the stratosphere (tough liquid part of the outer mantle) and the lithosphere (the stiffer outer mantle and the crust). Because of the intense pressure and temperature in the mantle convection currents occur. To learn about what influence t ...
Heat wave

A heat wave is a prolonged period of excessively hot weather, which may be accompanied by high humidity, especially in oceanic climate countries. While definitions vary, a heat wave is measured relative to the usual weather in the area and relative to normal temperatures for the season. Temperatures that people from a hotter climate consider normal can be termed a heat wave in a cooler area if they are outside the normal climate pattern for that area.The term is applied both to routine weather variations and to extraordinary spells of heat which may occur only once a century. Severe heat waves have caused catastrophic crop failures, thousands of deaths from hyperthermia, and widespread power outages due to increased use of air conditioning. A heat wave is considered extreme weather, and a danger because heat and sunlight may overheat the human body.