Fundamentals of the Heat Transfer Theory
... In engineering practice it is important to know in each particular case the regularities of heat interaction between the surface of a considered body and its surrounding medium (heat transfer processes), i.e., it is necessary to know how to determine a surface temperature and a heat flux to the surf ...
... In engineering practice it is important to know in each particular case the regularities of heat interaction between the surface of a considered body and its surrounding medium (heat transfer processes), i.e., it is necessary to know how to determine a surface temperature and a heat flux to the surf ...
PHY2216: Tutorial Questions 5 TEMPERATURE 5.1 Temperature
... mass 1 kg is gently added to the mixture in the calorimeter and this causes all the ice just to melt. Calculate the initial temperature of the metal. (Specific heat capacity of water = 4.2 x 103 J kg-1 0C-1, specific heat capacity of the metal = 4.2 x 102 J kg-1 0C-1, specific latent heat of fusion ...
... mass 1 kg is gently added to the mixture in the calorimeter and this causes all the ice just to melt. Calculate the initial temperature of the metal. (Specific heat capacity of water = 4.2 x 103 J kg-1 0C-1, specific heat capacity of the metal = 4.2 x 102 J kg-1 0C-1, specific latent heat of fusion ...
Thermal Applications Category User Guide
... applications derive their geometrical data from the ModelBuilder. This is supplemented with application-specific data provided within the Thermal Application Category. The input data requirements of the thermal applications are summarised below. The data is managed by utility programs invoked from t ...
... applications derive their geometrical data from the ModelBuilder. This is supplemented with application-specific data provided within the Thermal Application Category. The input data requirements of the thermal applications are summarised below. The data is managed by utility programs invoked from t ...
HEAT TRANSFER AND THE SECOND LAW
... Thus far we’ve used the first law of thermodynamics: Energy is conserved. Where does the second law come in? One way is when heat flows. Heat flows in response to a temperature gradient. If two points are in thermal contact and at different temperatures, T1 and T2 then energy is transferred between ...
... Thus far we’ve used the first law of thermodynamics: Energy is conserved. Where does the second law come in? One way is when heat flows. Heat flows in response to a temperature gradient. If two points are in thermal contact and at different temperatures, T1 and T2 then energy is transferred between ...
basic concept of thermodynamics
... between the various units. • English system: It has no apparent systematic numerical base, and various units in this system are related to each other rather ...
... between the various units. • English system: It has no apparent systematic numerical base, and various units in this system are related to each other rather ...
KWL – Chapter 14
... A material that conducts heat well is called a _______________________________. o Metals such as silver and stainless steel are _________________ conductors. o A good conductor, such as a tile floor, will feel cool to the touch because it transfers heat away from your skin easily. A material tha ...
... A material that conducts heat well is called a _______________________________. o Metals such as silver and stainless steel are _________________ conductors. o A good conductor, such as a tile floor, will feel cool to the touch because it transfers heat away from your skin easily. A material tha ...
ENERGY, HEAT AND TEMPERATURE
... Latent heat is measured according to water’s response to absorbing or releasing energy. Water is the only substance that exists in all three ‘states of being’ at the same time at earth’s ambient temperature and air pressure. Solid (ice Liquid (water) Gas (water vapor) ...
... Latent heat is measured according to water’s response to absorbing or releasing energy. Water is the only substance that exists in all three ‘states of being’ at the same time at earth’s ambient temperature and air pressure. Solid (ice Liquid (water) Gas (water vapor) ...
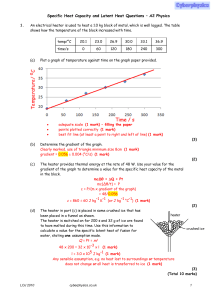
Specific Heat Capacity and Latent Heat Questions
... The heater in part (c) is placed in some crushed ice that has been placed in a funnel as shown. The heater is switched on for 200 s and 32 g of ice are found to have melted during this time. Use this information to calculate a value for the specific latent heat of fusion for water, stating one assum ...
... The heater in part (c) is placed in some crushed ice that has been placed in a funnel as shown. The heater is switched on for 200 s and 32 g of ice are found to have melted during this time. Use this information to calculate a value for the specific latent heat of fusion for water, stating one assum ...
Chapter 15 Solutions
... In the steam engine, the high-temperature reservoir is the heated, high-pressure steam from the boiler. The low-temperature reservoir is the condensed water in the condenser. ...
... In the steam engine, the high-temperature reservoir is the heated, high-pressure steam from the boiler. The low-temperature reservoir is the condensed water in the condenser. ...
Lecture 16-Chapter 7-October 19, 2005
... Enthalpy Changes Accompanying Changes in States of Matter. Calculate H for the process in which 50.0 g of water is converted from liquid at 10.0°C to vapor at 25.0°C. Break the problem into two steps: Raise the temperature of the liquid first then completely vaporize it. The total enthalpy change i ...
... Enthalpy Changes Accompanying Changes in States of Matter. Calculate H for the process in which 50.0 g of water is converted from liquid at 10.0°C to vapor at 25.0°C. Break the problem into two steps: Raise the temperature of the liquid first then completely vaporize it. The total enthalpy change i ...
binomial distribution ppt
... Points regarding the binomial distribution: • A binomial random variable is always discrete. • To graph use a probability histogram. • If p = 0.5 then the probability histogram will be bell-shaped. • If p < 0.5 then the probability histogram will be skewed right. • If p > 0.5 then the probability hi ...
... Points regarding the binomial distribution: • A binomial random variable is always discrete. • To graph use a probability histogram. • If p = 0.5 then the probability histogram will be bell-shaped. • If p < 0.5 then the probability histogram will be skewed right. • If p > 0.5 then the probability hi ...
Intro to Physics Lab
... temperature range between room temperature (water) and water boiling temperature (metal). To compare the accepted values of specific heat for 2 different metals ...
... temperature range between room temperature (water) and water boiling temperature (metal). To compare the accepted values of specific heat for 2 different metals ...
Unit B: Understanding Energy Conversion Technologies
... Later he reasoned that water must be composed of tiny unseen _______________. These particles are in _______________, ________________ motion. The motion of the pollen grains must be caused by ___________________ between the ____________________ and the other unseen ______________. (Brown was un ...
... Later he reasoned that water must be composed of tiny unseen _______________. These particles are in _______________, ________________ motion. The motion of the pollen grains must be caused by ___________________ between the ____________________ and the other unseen ______________. (Brown was un ...
Thermodynamics for Materials and Metallurgical Engineers
... Theory of heat was being debunked. In 1798 Count Rumford proposed that heat was not a fluid but rather a form of energy. Although Carnot lived in the world of controversy on these competing views of heat, the controversy, which ended with Joule’s work in 1840, it did not prevent Carnot’s progress. T ...
... Theory of heat was being debunked. In 1798 Count Rumford proposed that heat was not a fluid but rather a form of energy. Although Carnot lived in the world of controversy on these competing views of heat, the controversy, which ended with Joule’s work in 1840, it did not prevent Carnot’s progress. T ...
Conduction
... in. diameter are to be planted throughout the board, with a center to center distance of 0.06 in. Determine the new value of the thermal resistance of the epoxy board for heat conduction across its thickness as a result of this modification. A 50 m long section of a steam pipe whose outer diameter i ...
... in. diameter are to be planted throughout the board, with a center to center distance of 0.06 in. Determine the new value of the thermal resistance of the epoxy board for heat conduction across its thickness as a result of this modification. A 50 m long section of a steam pipe whose outer diameter i ...
File
... For a process in a simple calorimeter, the system is the chemical reaction, and the surroundings are the water and the calorimeter, so: qreaction = - (qcalorimeter+ qH2O) ...
... For a process in a simple calorimeter, the system is the chemical reaction, and the surroundings are the water and the calorimeter, so: qreaction = - (qcalorimeter+ qH2O) ...
Ch#3 Matter - Seattle Central College
... • The amount of energy stored in a material is its chemical potential energy. • The stored energy arises mainly from the attachments between atoms in the molecules and the attractive forces between molecules. • When materials undergo a physical change, the attractions between molecules change as the ...
... • The amount of energy stored in a material is its chemical potential energy. • The stored energy arises mainly from the attachments between atoms in the molecules and the attractive forces between molecules. • When materials undergo a physical change, the attractions between molecules change as the ...
Provedení, principy činnosti a základy výpočtu pro výměníky tepla
... Thermodynamic cycles Periodically repeating processes with working fluid (water, hydrocarbons, CO2,…) when heat is supplied to the fluid in the first phase of the process followed by the second phase of heat removal (final state of the working medium is the same as the initial one, therefore the cyc ...
... Thermodynamic cycles Periodically repeating processes with working fluid (water, hydrocarbons, CO2,…) when heat is supplied to the fluid in the first phase of the process followed by the second phase of heat removal (final state of the working medium is the same as the initial one, therefore the cyc ...
Worksheet Key - UCSB C.L.A.S.
... a. The system does work on the surroundings and w < 0 b. The surroundings do work on the system and w < 0 c. The system does work on the surroundings and w > 0 d. The surroundings do work on the system and w > 0 e. No work is done, w = 0 6. There are two containers, one has 1 mole of CO2 (g) and the ...
... a. The system does work on the surroundings and w < 0 b. The surroundings do work on the system and w < 0 c. The system does work on the surroundings and w > 0 d. The surroundings do work on the system and w > 0 e. No work is done, w = 0 6. There are two containers, one has 1 mole of CO2 (g) and the ...
... This paper investigates the thermal and hydraulic performance of two solar thermal mini-channel absorber plates for compact (thin and light-weight) solar thermal collectors. The proposed collectors will be an architecturally attractive option for incorporation in buildings and have the potential for ...
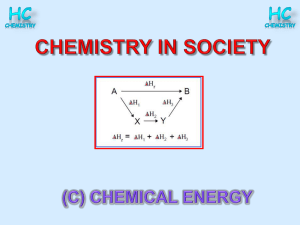
Enthalpy of combustion
... The enthalpy of combustion of a substance is the amount of energy given out when one mole of a substance burns in excess oxygen. ...
... The enthalpy of combustion of a substance is the amount of energy given out when one mole of a substance burns in excess oxygen. ...
2010 MULTIPHYSICS MODELING OF INDUCTION HARDENING OF RING GEARS A.Candeo HES
... The industry demands for high-quality gears, meeting severe specifications both in terms of performances (high strength, long life) and costs. This can be attained by combining a very hard, wear-resistant surface layer with a softer, tougher core. Case-hardening seems to represent nowadays the most ...
... The industry demands for high-quality gears, meeting severe specifications both in terms of performances (high strength, long life) and costs. This can be attained by combining a very hard, wear-resistant surface layer with a softer, tougher core. Case-hardening seems to represent nowadays the most ...
Cogeneration

Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time. Trigeneration or combined cooling, heat and power (CCHP) refers to the simultaneous generation of electricity and useful heating and cooling from the combustion of a fuel or a solar heat collector. Cogeneration is a thermodynamically efficient use of fuel. In separate production of electricity, some energy must be discarded as waste heat, but in cogeneration this thermal energy is put to use. All thermal power plants emit heat during electricity generation, which can be released into the natural environment through cooling towers, flue gas, or by other means. In contrast, CHP captures some or all of the by-product for heating, either very close to the plant, or—especially in Scandinavia and Eastern Europe—as hot water for district heating with temperatures ranging from approximately 80 to 130 °C. This is also called combined heat and power district heating (CHPDH). Small CHP plants are an example of decentralized energy. By-product heat at moderate temperatures (100–180 °C, 212–356 °F) can also be used in absorption refrigerators for cooling.The supply of high-temperature heat first drives a gas or steam turbine-powered generator and the resulting low-temperature waste heat is then used for water or space heating as described in cogeneration. At smaller scales (typically below 1 MW) a gas engine or diesel engine may be used. Trigeneration differs from cogeneration in that the waste heat is used for both heating and cooling, typically in an absorption refrigerator. CCHP systems can attain higher overall efficiencies than cogeneration or traditional power plants. In the United States, the application of trigeneration in buildings is called building cooling, heating and power (BCHP). Heating and cooling output may operate concurrently or alternately depending on need and system construction.Cogeneration was practiced in some of the earliest installations of electrical generation. Before central stations distributed power, industries generating their own power used exhaust steam for process heating. Large office and apartment buildings, hotels and stores commonly generated their own power and used waste steam for building heat. Due to the high cost of early purchased power, these CHP operations continued for many years after utility electricity became available.