Thermodynamics
... reaction, the temperature of the water changes to 31.0°C. The heat flow is calculated: qwater = 4.18 J/(g·°C) x 200 g x (31.0°C - 25.0°C) qwater = +5.0 x 103 J ...
... reaction, the temperature of the water changes to 31.0°C. The heat flow is calculated: qwater = 4.18 J/(g·°C) x 200 g x (31.0°C - 25.0°C) qwater = +5.0 x 103 J ...
Thermochemistry Energy - the capacity to do work Potential energy
... open system - exchanges matter and energy with the surroundings closed system - exchanges energy but not matter with the surroundings isolated system - exchanges neither energy nor matter with the surroundings Internal Energy (U) - the total energy contained within the system Heat (q) - the energy t ...
... open system - exchanges matter and energy with the surroundings closed system - exchanges energy but not matter with the surroundings isolated system - exchanges neither energy nor matter with the surroundings Internal Energy (U) - the total energy contained within the system Heat (q) - the energy t ...
HS 03 Solid and liquid insulating materials
... Liquids and solids have higher molecular densi5es than gases: ...
... Liquids and solids have higher molecular densi5es than gases: ...
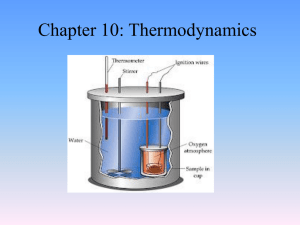
Specific Heat of a Metal
... 1. Since the specific heat of water is given in units of joules per gram degree Celsius why do we measure the volume of water in the calorimeter instead of its mass? 2. A 22.50 g piece of an unknown metal is heated to 100oC then transferred quickly and without cooling into 100.0 mL of water at 20.0o ...
... 1. Since the specific heat of water is given in units of joules per gram degree Celsius why do we measure the volume of water in the calorimeter instead of its mass? 2. A 22.50 g piece of an unknown metal is heated to 100oC then transferred quickly and without cooling into 100.0 mL of water at 20.0o ...
living with the lab
... conservation of energy Energy can change form, but it can’t be created or destroyed. Within an isolated system, energy is constant. ...
... conservation of energy Energy can change form, but it can’t be created or destroyed. Within an isolated system, energy is constant. ...
Relationships Between Heat and Work
... • As long as a substance does not change phase, its internal energy will increase as long as its temperature increases • Work can transfer energy to a substance – Results in an increase in internal energy • Can be transferred to another substance as heat ...
... • As long as a substance does not change phase, its internal energy will increase as long as its temperature increases • Work can transfer energy to a substance – Results in an increase in internal energy • Can be transferred to another substance as heat ...
Heat stress round table
... implement a specific strategy during the carry over period? • What is, from your point of view, the main limiting factor to improve conception rate during the heat stress period? ...
... implement a specific strategy during the carry over period? • What is, from your point of view, the main limiting factor to improve conception rate during the heat stress period? ...
Analysis of Heat Transfer in Rectangular
... θ = Temperature difference, [k] Subscripts conv = convection ch = channel sp = single phase bot = bottom ƒ = fluid i = inlet o =outlet Micro-channel Heat transfer has the very potential of wide applications in cooling high power density microchips in the CPU system, the micro power systems and even ...
... θ = Temperature difference, [k] Subscripts conv = convection ch = channel sp = single phase bot = bottom ƒ = fluid i = inlet o =outlet Micro-channel Heat transfer has the very potential of wide applications in cooling high power density microchips in the CPU system, the micro power systems and even ...
Page 1 of 2 Gerbing`s Heated Clothing // How it Works 02/11/2009
... further tune how the heat is delivered. More, when using the ribbon matrix, we could refine the heat delivery to an even greater degree by altering the number of wires in the ribbon (from 2 up to 6). It is this “tunability” to each garment application that is one of the major advantages of Microwire ...
... further tune how the heat is delivered. More, when using the ribbon matrix, we could refine the heat delivery to an even greater degree by altering the number of wires in the ribbon (from 2 up to 6). It is this “tunability” to each garment application that is one of the major advantages of Microwire ...
Ch.19 (section 1 only)
... Device that uses heat to perform work Hot Reservoir (e.g. steam) Cool Reservoir (e.g. pool of water) Efficiency is work done per unit of input heat (e = W/QH) • Ex. A heat engine does 100J of work when given 300J from the hot reservoir. The efficiency is 100J/300J = 0.33 = ...
... Device that uses heat to perform work Hot Reservoir (e.g. steam) Cool Reservoir (e.g. pool of water) Efficiency is work done per unit of input heat (e = W/QH) • Ex. A heat engine does 100J of work when given 300J from the hot reservoir. The efficiency is 100J/300J = 0.33 = ...
Ch 10 Review activity
... 4. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67.4 kJ of heat, and its temperature changes from 32°C to 57°C. When this piece of wood is burned, would the combustion reaction be endothermic or exothermic? Where does the energy that would be transferred in this re ...
... 4. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67.4 kJ of heat, and its temperature changes from 32°C to 57°C. When this piece of wood is burned, would the combustion reaction be endothermic or exothermic? Where does the energy that would be transferred in this re ...
Chap19Class2
... 19-5 Latent Heat The latent heat of vaporization is relevant for evaporation as well as boiling. The heat of vaporization of water rises slightly as the temperature decreases. On a molecular level, the heat added during a change of state does not increase the kinetic energy of individual molecules, ...
... 19-5 Latent Heat The latent heat of vaporization is relevant for evaporation as well as boiling. The heat of vaporization of water rises slightly as the temperature decreases. On a molecular level, the heat added during a change of state does not increase the kinetic energy of individual molecules, ...
D12E12Safety1\4Curr\emet
... 7.2.6 define kinetic energy as energy stored in molecules by virtue of their velocity; kinetic energy has a value of V2 (i.e. 0.5 of velocity squared) per unit mass of substance 7.2.7 state that energy in transition between bodies or systems can only be heat flow (or heat transfer) (Q) and work flow ...
... 7.2.6 define kinetic energy as energy stored in molecules by virtue of their velocity; kinetic energy has a value of V2 (i.e. 0.5 of velocity squared) per unit mass of substance 7.2.7 state that energy in transition between bodies or systems can only be heat flow (or heat transfer) (Q) and work flow ...
Review Part 2
... Directions: For questions #3-7, identify the following as a solid, liquid or gas. 3. Lowest amount of kinetic energy (particle movement). _______________ 4. Definite volume and definite shape (shape and volume stay the same). ________________ 5. Particles are farthest apart and move independently of ...
... Directions: For questions #3-7, identify the following as a solid, liquid or gas. 3. Lowest amount of kinetic energy (particle movement). _______________ 4. Definite volume and definite shape (shape and volume stay the same). ________________ 5. Particles are farthest apart and move independently of ...
RTF
... What is the relationship between the amount of kinetic energy a particle has and how fast it is moving? Pick one of the following: A. Slower moving particles have more kinetic energy than faster particles. ...
... What is the relationship between the amount of kinetic energy a particle has and how fast it is moving? Pick one of the following: A. Slower moving particles have more kinetic energy than faster particles. ...
4. One mole of a monatomic ideal gas initially at temperature 0 T
... c. (10) Assume the radius shrinks by a factor of 2 so R′ = R / 2 while the mass remains the same. Find the relative change in the gravitational and electron energies (assuming both relativistic and non‐relativistic) between R and R’. d. (10) Discuss whether the star is more stable (less likely to co ...
... c. (10) Assume the radius shrinks by a factor of 2 so R′ = R / 2 while the mass remains the same. Find the relative change in the gravitational and electron energies (assuming both relativistic and non‐relativistic) between R and R’. d. (10) Discuss whether the star is more stable (less likely to co ...
Heat - Denton ISD
... The total heat involved during a phase change depends on the latent heat, L and on the total mass of the substance: Q = mL. (Q is the heat required or given off during the phase change. ...
... The total heat involved during a phase change depends on the latent heat, L and on the total mass of the substance: Q = mL. (Q is the heat required or given off during the phase change. ...