FSK Shield - Fi-Foil
... temperatures upward to 150 degrees or higher. These higher temperatures will increase the heat gain in your air con-ditioning ducts and reduce the performance of mass insulation (the R-values of mass insulation are determined at 75oF - higher temperatures lowers the R-value). In addition, the extrem ...
... temperatures upward to 150 degrees or higher. These higher temperatures will increase the heat gain in your air con-ditioning ducts and reduce the performance of mass insulation (the R-values of mass insulation are determined at 75oF - higher temperatures lowers the R-value). In addition, the extrem ...
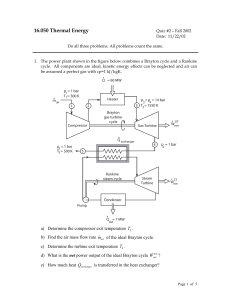
16.050 Thermal Energy
... f) What is the net power output of the Rankine cycle W&netST ? g) What is the overall thermal efficiency of the combined cycle? 2. The sketch below shows a perfectly insulated container with two compartments separated by a non-adiabatic, frictionless piston. Both compartments are at the same pressu ...
... f) What is the net power output of the Rankine cycle W&netST ? g) What is the overall thermal efficiency of the combined cycle? 2. The sketch below shows a perfectly insulated container with two compartments separated by a non-adiabatic, frictionless piston. Both compartments are at the same pressu ...
Nernst`s postulate derived directly from the vanishing heat capacity
... It is worthwhile pointing out that when T is very small, so is −(∆T )S , whereas (∆yi )S need not be small. It may have a finite value which can be varied by controlling the exterior conditions, whether the temperature of the system is high or low [1,2]. In fact, this observation has been used extens ...
... It is worthwhile pointing out that when T is very small, so is −(∆T )S , whereas (∆yi )S need not be small. It may have a finite value which can be varied by controlling the exterior conditions, whether the temperature of the system is high or low [1,2]. In fact, this observation has been used extens ...
AS90184 - NBCPhyyear11
... heat to travel through it, a thermal conductor. Convection: Within a fluid when hot material is less dense and it rises, cooler material get heated by the source and rises up Radiation: By being emitted from the source directly as heat energy, travels through space in all directions, by infra red li ...
... heat to travel through it, a thermal conductor. Convection: Within a fluid when hot material is less dense and it rises, cooler material get heated by the source and rises up Radiation: By being emitted from the source directly as heat energy, travels through space in all directions, by infra red li ...
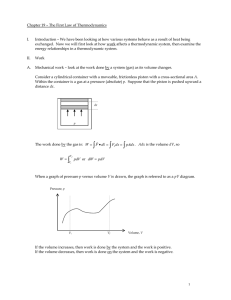
Chapter 19 – The First Law of Thermodynamics
... Add heat to the system and keep the temperature constant. Where does the energy go? (Remember that the temperature is directly proportional to the translational kinetic energy, TKE.) ...
... Add heat to the system and keep the temperature constant. Where does the energy go? (Remember that the temperature is directly proportional to the translational kinetic energy, TKE.) ...
GAS LAWS AND KINETIC THEORY
... 0th Law: (Thermal Equilibrium) If two objects are in thermal equilibrium with a third object they are also in thermal equilibrium with each other. The internal energy is de ned as the total energy within the system. If no phase changes are involved the internal energy is proportional to the temperat ...
... 0th Law: (Thermal Equilibrium) If two objects are in thermal equilibrium with a third object they are also in thermal equilibrium with each other. The internal energy is de ned as the total energy within the system. If no phase changes are involved the internal energy is proportional to the temperat ...
Chemistry - Scarsdale Schools
... 7. A 3.50 g sample of nickel metal at a temperature of 88.0oC is placed into a 50.0 g sample of water at 25.0oC. What will be the final temperature of both the metal and water after “thermal equilibrium” is reached? The specific heat capacity of nickel is 0.444 J/goC. ...
... 7. A 3.50 g sample of nickel metal at a temperature of 88.0oC is placed into a 50.0 g sample of water at 25.0oC. What will be the final temperature of both the metal and water after “thermal equilibrium” is reached? The specific heat capacity of nickel is 0.444 J/goC. ...
temperature.
... • DEVELOP: In energy balance, the rate of energy arriving per square meter (240 W/m2) equals the rate going out, namely e T4 W/m2. Equating the two with e = 1 gives 240 W/m2 = T4. ...
... • DEVELOP: In energy balance, the rate of energy arriving per square meter (240 W/m2) equals the rate going out, namely e T4 W/m2. Equating the two with e = 1 gives 240 W/m2 = T4. ...
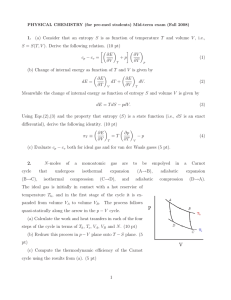
First Law of Thermodynamics
... system (its particular properties T,V,P, ...) not how the system arrived in this state. Changes in internal energy are then associated with any energy that flows into or out of the system. We discussed two forms of energy flow: heat and temperature Heat. Heat is not temperature. Can't say this enoug ...
... system (its particular properties T,V,P, ...) not how the system arrived in this state. Changes in internal energy are then associated with any energy that flows into or out of the system. We discussed two forms of energy flow: heat and temperature Heat. Heat is not temperature. Can't say this enoug ...
Thermodynamics test
... is the process of passing from the solid phase directly into the vapor phase. ...
... is the process of passing from the solid phase directly into the vapor phase. ...
Modeling of combined thermal and mechanical action in roller
... A combined thermal and mechanical action in roller compacted concrete (RCC) dam analysis is carried out using a three-dimensional finite element method. In this work a numerical procedure for the simulation of construction process and service life of RCC dams is presented. It takes into account the ...
... A combined thermal and mechanical action in roller compacted concrete (RCC) dam analysis is carried out using a three-dimensional finite element method. In this work a numerical procedure for the simulation of construction process and service life of RCC dams is presented. It takes into account the ...
Homework_bOLIDE
... e) Was the energy generated in the creation of the Earth larger or smaller than that for the moon? How much more, or less, is the Moons accretion energy relative to the Earth? What can you infer about the thermal history of the two planets from these differences (thinking about our discussion in cla ...
... e) Was the energy generated in the creation of the Earth larger or smaller than that for the moon? How much more, or less, is the Moons accretion energy relative to the Earth? What can you infer about the thermal history of the two planets from these differences (thinking about our discussion in cla ...
Work, YA!!!!!! Finally something easy
... Sometimes we waste our energy doing work when we’re not parallel to the object we wish to work on. Make sense? EX Pushing a box at an angle means the box moves to the direction wanted but some work is wasted up or down, as our desired outcome is to move the box left to right. ...
... Sometimes we waste our energy doing work when we’re not parallel to the object we wish to work on. Make sense? EX Pushing a box at an angle means the box moves to the direction wanted but some work is wasted up or down, as our desired outcome is to move the box left to right. ...
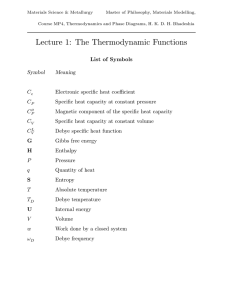
Thermodynamic functions - Phase Transformations Group
... The Helmholtz free energy F is the corresponding term at constant volume, when H is replaced by U in equation 9. A process can occur spontaneously if it leads to a reduction in the free energy. Quantities such as H, G and S are path independent and therefore are called functions of state. More About ...
... The Helmholtz free energy F is the corresponding term at constant volume, when H is replaced by U in equation 9. A process can occur spontaneously if it leads to a reduction in the free energy. Quantities such as H, G and S are path independent and therefore are called functions of state. More About ...
Calorimetry worksheet - MRS. STOTTS CHEMISTRY
... There are several terms used in this chapter that sound very similar. Use the data provided to calculate each of them to clarify the differences. I’ve added some “Notes” that I hope will help. 74.8 J of heat is required to raise the temperature of 18.69 g of silver from 10.0C to 27.0C. a. What is ...
... There are several terms used in this chapter that sound very similar. Use the data provided to calculate each of them to clarify the differences. I’ve added some “Notes” that I hope will help. 74.8 J of heat is required to raise the temperature of 18.69 g of silver from 10.0C to 27.0C. a. What is ...