heat engine
... In general, irreversible processes cause us to lose some, but not necessarily all, of the ability to do work. This partial loss can be expressed in terms of a concept called entropy. ...
... In general, irreversible processes cause us to lose some, but not necessarily all, of the ability to do work. This partial loss can be expressed in terms of a concept called entropy. ...
Slide 1 - KaiserScience
... This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permit ...
... This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permit ...
Ch15Thermo (1)
... Air pollution is also emitted by power plants, industries, and consumers. Some of this pollution results in a buildup of CO2 in the atmosphere, contributing to global warming. This can be minimized through careful choices of fuels and processes. ...
... Air pollution is also emitted by power plants, industries, and consumers. Some of this pollution results in a buildup of CO2 in the atmosphere, contributing to global warming. This can be minimized through careful choices of fuels and processes. ...
Slide 1
... Air pollution is also emitted by power plants, industries, and consumers. Some of this pollution results in a buildup of CO2 in the atmosphere, contributing to global warming. This can be minimized through careful choices of fuels and processes. ...
... Air pollution is also emitted by power plants, industries, and consumers. Some of this pollution results in a buildup of CO2 in the atmosphere, contributing to global warming. This can be minimized through careful choices of fuels and processes. ...
Homework 6: Heat Transfer (Lowrie Chapter 4.2)
... (b) Estimate the average rate of heat production H in the crust beneath the above location. (c) At what rate is heat flowing from the underlying mantle into the crust?, i.e., what is Q(z=35) km? Briefly explain why the difference between q0 and q(z=35) does (or does not) make sense given your estima ...
... (b) Estimate the average rate of heat production H in the crust beneath the above location. (c) At what rate is heat flowing from the underlying mantle into the crust?, i.e., what is Q(z=35) km? Briefly explain why the difference between q0 and q(z=35) does (or does not) make sense given your estima ...
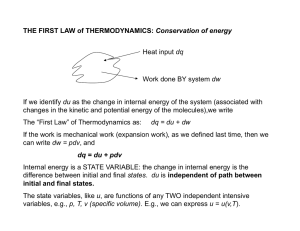
THE FIRST LAW of THERMODYNAMICS: Conservation of energy
... Since h = u + Pv and, for an ideal gas, Pv = RT, then for the ideal gas h = u + RT R is constant, and u = u(T) only, so we conclude h = h(T) only for an ideal gas. Aside: application of enthalpy balance in steady-flow system: energy balance for a single-stream, steady-flow system with negligible ch ...
... Since h = u + Pv and, for an ideal gas, Pv = RT, then for the ideal gas h = u + RT R is constant, and u = u(T) only, so we conclude h = h(T) only for an ideal gas. Aside: application of enthalpy balance in steady-flow system: energy balance for a single-stream, steady-flow system with negligible ch ...
Thermodynamics - Clayton State University
... Heat energy is based on random movement of molecules, so it is a disorganized form of energy. It is much easier to produce disorganized motion than organized motion, and it is much more difficult to get the full potential out of heat energy, since efficiency would require that the particles move in ...
... Heat energy is based on random movement of molecules, so it is a disorganized form of energy. It is much easier to produce disorganized motion than organized motion, and it is much more difficult to get the full potential out of heat energy, since efficiency would require that the particles move in ...
05Thermal_PhysicsALT
... different states and amounts of heat or work to go between them. • Heat Transfer: deals with the rate at which energy (as heat) is transferred. • Three modes of heat transfer: – Conduction: propagation of vibrations through adjacent atoms. – Convection: energy carried by actual transfer of mass. – R ...
... different states and amounts of heat or work to go between them. • Heat Transfer: deals with the rate at which energy (as heat) is transferred. • Three modes of heat transfer: – Conduction: propagation of vibrations through adjacent atoms. – Convection: energy carried by actual transfer of mass. – R ...
Density of Thermal Insulating Materials Kg/m3 K
... reaching up to 1.00. This happens in summer where the heat flow is from the upward direction towards downwards. This improvement in thermal resistance is due to a trapped air cavity up to 60 mm in width. This value is achieved by using a single aluminum foil as a reflective membrane, provided that i ...
... reaching up to 1.00. This happens in summer where the heat flow is from the upward direction towards downwards. This improvement in thermal resistance is due to a trapped air cavity up to 60 mm in width. This value is achieved by using a single aluminum foil as a reflective membrane, provided that i ...
Page 93 Static electricity is the buildup of electric charge on an
... A load is a device in a circuit that changes electrical energy into other kinds of energy. Ex. lightbulb The wires of a circuit are used to connect energy sources to loads. Circuits may have several loads and many wires, but often only have one energy source. ...
... A load is a device in a circuit that changes electrical energy into other kinds of energy. Ex. lightbulb The wires of a circuit are used to connect energy sources to loads. Circuits may have several loads and many wires, but often only have one energy source. ...
List 6-10 types of energy and give an example of each. State
... The sun shines down as an input of radiant (KE), which is then transformed into chemical (PE) as the sunflower produces a sunflower seed. 3. What is heat? How are heat and temperature different? What direction does heat transfer? [Heat & Thermal Energy Part 1: Kinetic Molecular Theory and Heat] Heat ...
... The sun shines down as an input of radiant (KE), which is then transformed into chemical (PE) as the sunflower produces a sunflower seed. 3. What is heat? How are heat and temperature different? What direction does heat transfer? [Heat & Thermal Energy Part 1: Kinetic Molecular Theory and Heat] Heat ...
Atomic Structure
... broken down and oxidized (has oxygen added), and there is energy released. c. We gain energy from the food, air and water that we take in. This energy is converted to heat and into work, and stored as potential energy, for example in fats. The total energy is always conserved, and the change in inte ...
... broken down and oxidized (has oxygen added), and there is energy released. c. We gain energy from the food, air and water that we take in. This energy is converted to heat and into work, and stored as potential energy, for example in fats. The total energy is always conserved, and the change in inte ...
floor level coverage charts
... radiant pattern. Since concrete is a very good conductor of heat, a reasonably uniform floor temperature eventually will be reached. The amount of time it takes to reach “thermal equilibrium” is in direct correlation to the distance between the heaters. B. Location In most applications, 70 to 80 per ...
... radiant pattern. Since concrete is a very good conductor of heat, a reasonably uniform floor temperature eventually will be reached. The amount of time it takes to reach “thermal equilibrium” is in direct correlation to the distance between the heaters. B. Location In most applications, 70 to 80 per ...
Relation between local temperature gradients and the direction of
... These systems can not only dissipate energy in the form of heat, but can also pump energy between the different reservoirs, generating refrigeration. We have defined a ‘‘local’’ temperature along these set ups by introducing a thermometer, i.e. a macroscopic system which is in local equilibrium with ...
... These systems can not only dissipate energy in the form of heat, but can also pump energy between the different reservoirs, generating refrigeration. We have defined a ‘‘local’’ temperature along these set ups by introducing a thermometer, i.e. a macroscopic system which is in local equilibrium with ...
Document
... The Nature of Energy (cont.) • Chemical potential energy is energy stored in a substance because of its composition. • Chemical potential energy is important in chemical reactions. • Heat is energy that is in the process of flowing (transferring) from a warmer object to a cooler object. • q is used ...
... The Nature of Energy (cont.) • Chemical potential energy is energy stored in a substance because of its composition. • Chemical potential energy is important in chemical reactions. • Heat is energy that is in the process of flowing (transferring) from a warmer object to a cooler object. • q is used ...
Energy & Power
... • Work can be changed into heat relatively easily – the reverse is a different challenge • From the second law of thermodynamics, we know that : – it is not possible to change completely into work, with no other change taking place ...
... • Work can be changed into heat relatively easily – the reverse is a different challenge • From the second law of thermodynamics, we know that : – it is not possible to change completely into work, with no other change taking place ...
Heat
... This is Figure 17 from page 394 from the book As heat is added, temperature increases linearly (q=nCT) Except, when a phase change occurs, because all the heat is going into changing the phase (q=m L, note no T) ...
... This is Figure 17 from page 394 from the book As heat is added, temperature increases linearly (q=nCT) Except, when a phase change occurs, because all the heat is going into changing the phase (q=m L, note no T) ...