Chapter 6
... An environmental chemist analyzed the effluent (waste) from an industrial process known to produce the compounds carbon tetrachloride (CCl4) and benzoic acid (HC7H5O2), a weak acid that has one acidic hydrogen atom per molecule. A sample of the effluent weighing 0.3518 g was shaken with water, and t ...
... An environmental chemist analyzed the effluent (waste) from an industrial process known to produce the compounds carbon tetrachloride (CCl4) and benzoic acid (HC7H5O2), a weak acid that has one acidic hydrogen atom per molecule. A sample of the effluent weighing 0.3518 g was shaken with water, and t ...
Solubility
... In studio 5c, you dissolved salts to make hot and cold packs. For example: NaCl(s) → Na+(aq) + Cl-(aq) Hrxn = 4 kJ/mol All of the salt you added to the water dissolved so these reactions could be described as going to completion. Could you just keep dissolving sodium chloride in water to generate a ...
... In studio 5c, you dissolved salts to make hot and cold packs. For example: NaCl(s) → Na+(aq) + Cl-(aq) Hrxn = 4 kJ/mol All of the salt you added to the water dissolved so these reactions could be described as going to completion. Could you just keep dissolving sodium chloride in water to generate a ...
Practice Toxins Mid-Unit Test 08-09
... ______1. What type of reaction is this? Ag (s) + CuI2 (aq) AgI (s) + Cu(s) (A) single displacement (B) double displacement (C) combination reaction (D) decomposition reaction ______2.Calcium Chloride is abbreviated (A) CaCl (C) Ca2Cl (B) CaCl2 (D) Cl2Ca ______3. What is the molarity of 3.5 moles o ...
... ______1. What type of reaction is this? Ag (s) + CuI2 (aq) AgI (s) + Cu(s) (A) single displacement (B) double displacement (C) combination reaction (D) decomposition reaction ______2.Calcium Chloride is abbreviated (A) CaCl (C) Ca2Cl (B) CaCl2 (D) Cl2Ca ______3. What is the molarity of 3.5 moles o ...
REACTION PREDICTION
... Formation of a precipitate: A precipitate is an insoluble substance formed by the reaction of two aqueous substances. Two ions bond together so strongly that water can not pull them apart. Ex. Solutions of silver nitrate and lithium bromide are mixed. AgNO3(aq) + LiBr(aq) AgBr(s) + LiNO3(aq) Format ...
... Formation of a precipitate: A precipitate is an insoluble substance formed by the reaction of two aqueous substances. Two ions bond together so strongly that water can not pull them apart. Ex. Solutions of silver nitrate and lithium bromide are mixed. AgNO3(aq) + LiBr(aq) AgBr(s) + LiNO3(aq) Format ...
X273/13/02
... your pencil, put a horizontal line in the space provided (see sample question below). 7 There is only one correct answer to each question. 8 Any rough working should be done on the question paper or the rough working sheet, not on ...
... your pencil, put a horizontal line in the space provided (see sample question below). 7 There is only one correct answer to each question. 8 Any rough working should be done on the question paper or the rough working sheet, not on ...
balancing chemical equations worksheet
... b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following statements and write balanced chemical equations. The first has been completed as an example Example grey sodium me ...
... b. Chemists and other scientists always balance chemical equations. Please explain why this is so important. (Hint, refer to the law of conservation of mass) PART B, read the following statements and write balanced chemical equations. The first has been completed as an example Example grey sodium me ...
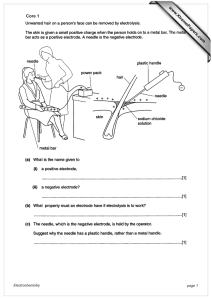
Core 1 www.XtremePapers.com Electrochemistry page 1
... does not conduct electricity (to operator) / plastic is an insulator / so operator does not get an electric shock ...
... does not conduct electricity (to operator) / plastic is an insulator / so operator does not get an electric shock ...
AP Chem Summer Assign Gen Chem Rev Problems
... b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to completely react with 45 grams of lithium hydroxide at STP? d. How many grams of lithium hydroxide is required to produce 25 g of lithium carbonate? e. How many moles of water ...
... b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to completely react with 45 grams of lithium hydroxide at STP? d. How many grams of lithium hydroxide is required to produce 25 g of lithium carbonate? e. How many moles of water ...
unit 6 - writing and balancing chemical equations
... 13. lithium phosphate solution is mixed with cesium iodide solution 14. acetic acid neutralizes a solution of strontium hydroxide 15. bubbles of hydrogen gas and a solution of strontium chloride are produced when metallic strontium is dropped into hydrochloric acid 16. a sample of mercury (I) oxide ...
... 13. lithium phosphate solution is mixed with cesium iodide solution 14. acetic acid neutralizes a solution of strontium hydroxide 15. bubbles of hydrogen gas and a solution of strontium chloride are produced when metallic strontium is dropped into hydrochloric acid 16. a sample of mercury (I) oxide ...
Preparation of spherical DDNP study Liu off on a journey
... Influence of ammonia salt crystals diazotization due in ammonia salt filtration process Amount drained liquor, ammonium salt crystals when stout, filtration fast, do not And then washed with water. Because the sodium picramate reduction is an exothermic reaction, the addition point may be scattered ...
... Influence of ammonia salt crystals diazotization due in ammonia salt filtration process Amount drained liquor, ammonium salt crystals when stout, filtration fast, do not And then washed with water. Because the sodium picramate reduction is an exothermic reaction, the addition point may be scattered ...
Unit C3, C3.1
... When the Russian chemist Dimitri Mendeleev put forward his periodic table in 1869, the atomic structure of elements was unknown. Mendeleev tried to arrange the elements in a meaningful way based on their chemical reactions. First he put the elements in order of their increasing atomic weight. He the ...
... When the Russian chemist Dimitri Mendeleev put forward his periodic table in 1869, the atomic structure of elements was unknown. Mendeleev tried to arrange the elements in a meaningful way based on their chemical reactions. First he put the elements in order of their increasing atomic weight. He the ...
AP Chemistry Summer Assignment
... ‘X’. Name the compound. 64. When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a. Write the balanced chemical equation for this reaction. b. How many liters of sulfur dioxide would be produced from 4.0 l of Oxygen? Assume 100% yield and that all gases are ...
... ‘X’. Name the compound. 64. When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a. Write the balanced chemical equation for this reaction. b. How many liters of sulfur dioxide would be produced from 4.0 l of Oxygen? Assume 100% yield and that all gases are ...
AP Chemistry Summer Assignment
... 33.Name the types of general inorganic reactions with example of each? 34.Define Acid, base and salt? Give some examples of each. 35.What mass of copper is required to replace silver from 4.00g of silver nitrate dissolved in ...
... 33.Name the types of general inorganic reactions with example of each? 34.Define Acid, base and salt? Give some examples of each. 35.What mass of copper is required to replace silver from 4.00g of silver nitrate dissolved in ...
General Chemistry Review Problems
... b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to completely react with 45 grams of lithium hydroxide at STP? d. How many grams of lithium hydroxide is required to produce 25 g of lithium carbonate? e. How many moles of water ...
... b. If 4 moles of LiOH reacts, how many moles of water will be produced? c. How many liters of carbon dioxide are required to completely react with 45 grams of lithium hydroxide at STP? d. How many grams of lithium hydroxide is required to produce 25 g of lithium carbonate? e. How many moles of water ...
AP Chemistry Summer Assignment
... element ‘X’. Name the compound. 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of sulfur dioxide would be produced from 10.0 l of Oxygen? Assume 100% yield and that all gase ...
... element ‘X’. Name the compound. 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of sulfur dioxide would be produced from 10.0 l of Oxygen? Assume 100% yield and that all gase ...
Chemistry@YIA – additional information
... What mass of hydrogen is produced when 192 g of magnesium is reacted with hydrochloric acid? Mg + 2HCl MgCl2 + H2 2. Draw a table and fill in the values from the question: ...
... What mass of hydrogen is produced when 192 g of magnesium is reacted with hydrochloric acid? Mg + 2HCl MgCl2 + H2 2. Draw a table and fill in the values from the question: ...
unit 7 h chem notes - chemical equations
... 1. The materials which you can start with are written first, and are called reactants. If there is more than one reactant, a plus (+) sign separates each individual reactant. Example: Sodium reacts with chlorine to form sodium chloride (NaCl). An arrow is written after the reactants.This is how the ...
... 1. The materials which you can start with are written first, and are called reactants. If there is more than one reactant, a plus (+) sign separates each individual reactant. Example: Sodium reacts with chlorine to form sodium chloride (NaCl). An arrow is written after the reactants.This is how the ...
Household Acids and Bases Lab
... A visual indicator is a chemical substance that reflects the nature of the chemical system in which it is placed by changing color. Most visual indicators are complex organic molecules that exist in multiple colored forms, one of which could be colorless, depending on the chemical environment. Many ...
... A visual indicator is a chemical substance that reflects the nature of the chemical system in which it is placed by changing color. Most visual indicators are complex organic molecules that exist in multiple colored forms, one of which could be colorless, depending on the chemical environment. Many ...
Lecture 11 - U of L Class Index
... medieval alchemy. To alchemists, any solid substance that did not melt and was not changed by fire into another substance was called an ‘‘earth.’’ Various compounds of Group 1 and 2 elements that were known in those times, such as NaOH and CaO, were alkaline according to the experimental tests of th ...
... medieval alchemy. To alchemists, any solid substance that did not melt and was not changed by fire into another substance was called an ‘‘earth.’’ Various compounds of Group 1 and 2 elements that were known in those times, such as NaOH and CaO, were alkaline according to the experimental tests of th ...
Sherbert
... bicarbonate. When the citric acid and sodium bicarbonate touch your saliva, they react together to make bubbles that fizz and pop in your mouth. The icing sugar and lollipop make the nice taste. This reaction is similar to the more common reaction between vinegar and sodium bicarbonate. When an a ...
... bicarbonate. When the citric acid and sodium bicarbonate touch your saliva, they react together to make bubbles that fizz and pop in your mouth. The icing sugar and lollipop make the nice taste. This reaction is similar to the more common reaction between vinegar and sodium bicarbonate. When an a ...
Chapter 4
... solution is to be standardized. 1.3009 M of KHP (potassium hydrogen phthalate, KHC8H4O4) is used as the titrant. KHP has one acidic hydrogen. 41.20 mL of the KHP solution is used to titrate the sodium hydroxide solution to the endpoint. What is the resulting concentration of the ...
... solution is to be standardized. 1.3009 M of KHP (potassium hydrogen phthalate, KHC8H4O4) is used as the titrant. KHP has one acidic hydrogen. 41.20 mL of the KHP solution is used to titrate the sodium hydroxide solution to the endpoint. What is the resulting concentration of the ...
Cl -1
... 5. Oxygen has an oxidation number of -2 unless it is combined with F (when it is +2), or it is in a peroxide (such as H2O2 or Na2O2), when it is -1. 6. The oxidation state of hydrogen in most of its compounds is +1 unless it is combined with a metal, in which case it is -1. ...
... 5. Oxygen has an oxidation number of -2 unless it is combined with F (when it is +2), or it is in a peroxide (such as H2O2 or Na2O2), when it is -1. 6. The oxidation state of hydrogen in most of its compounds is +1 unless it is combined with a metal, in which case it is -1. ...
Tertiary Treatment: Nutrient Removal, Filtration,and
... • A series of alternating aerobic and anoxic stages • Reduces the amount of air needed • No need for supplemental carbon source ...
... • A series of alternating aerobic and anoxic stages • Reduces the amount of air needed • No need for supplemental carbon source ...
Sodium hypochlorite

Sodium hypochlorite is a chemical compound with the formula NaClO. It is composed of a sodium cation (Na+) and a hypochlorite anion (ClO−); it may also be viewed as the sodium salt of hypochlorous acid. When dissolved in water it is commonly known as bleach, or liquid bleach. Sodium hypochlorite is practically and chemically distinct from chlorine. Sodium hypochlorite is frequently used as a disinfectant or a bleaching agent.