Internal Assessment: Fermentation Biology Higher Level
... molecules, there are more heat energy released and, as a result more molecules will collide with one another and with the enzymes in yeast. However, we cannot generalize ...
... molecules, there are more heat energy released and, as a result more molecules will collide with one another and with the enzymes in yeast. However, we cannot generalize ...
CO2 and O2 Distribution in Rubisco Suggests the
... specificity factor and turnover rate11,12 (Vc or kcat for carboxylation) with bacteria displaying low specificities and high turnover numbers, while plants have high specificities coupled to low turnover numbers. CO2 also plays a role in regulation of Rubisco activity. To be functional, Rubisco require ...
... specificity factor and turnover rate11,12 (Vc or kcat for carboxylation) with bacteria displaying low specificities and high turnover numbers, while plants have high specificities coupled to low turnover numbers. CO2 also plays a role in regulation of Rubisco activity. To be functional, Rubisco require ...
Stoichiometric Calculations
... 16 What is the largest number of of Li3 N formula units that could result from reacting 6 N2 molecules? 6 Li (s) + N2 (g) --# 2 Li3 N (s) ...
... 16 What is the largest number of of Li3 N formula units that could result from reacting 6 N2 molecules? 6 Li (s) + N2 (g) --# 2 Li3 N (s) ...
13C-NMR study of acid dissociation constant (pKa) effects on the
... spin−lattice relaxation time, the holding time between scans was set at 1 min. The data of 64 scans ...
... spin−lattice relaxation time, the holding time between scans was set at 1 min. The data of 64 scans ...
Gas phase infrared spectra of nonaromatic amino acids
... of ab initio calculations of harmonic and anharmonic vibrational frequencies [1,2]. In addition, gas phase spectra can be compared with spectra in liquids, solutions and matrix isolation, in order to assess the interaction with the solvent and neighboring molecules [3–9]. Biomolecules, like amino ac ...
... of ab initio calculations of harmonic and anharmonic vibrational frequencies [1,2]. In addition, gas phase spectra can be compared with spectra in liquids, solutions and matrix isolation, in order to assess the interaction with the solvent and neighboring molecules [3–9]. Biomolecules, like amino ac ...
Hazards and Precautions FORMALDEHIDE Formaldehyde
... (2 ppm) is quickly irritating to the eyes, and 20 ppm can cause permanent clouding of the cornea after only one exposure. Formaldehyde is also a sensitizing agent. Subsequent exposures can produce symptoms more quickly and at lower concentrations. Symptoms of exposure may include coughing, eye or sk ...
... (2 ppm) is quickly irritating to the eyes, and 20 ppm can cause permanent clouding of the cornea after only one exposure. Formaldehyde is also a sensitizing agent. Subsequent exposures can produce symptoms more quickly and at lower concentrations. Symptoms of exposure may include coughing, eye or sk ...
Identification of Unknown Bacterial Species - Katie Davis
... that the organism is MR positive. If Vogues-Proskauer reagents are added and a red color is formed, that indicates that the organism is VP positive (3). My organism was VP positive and MR negative because the media turned red only when Vogues Proskauer reagent was added. The nitrate broth contains ...
... that the organism is MR positive. If Vogues-Proskauer reagents are added and a red color is formed, that indicates that the organism is VP positive (3). My organism was VP positive and MR negative because the media turned red only when Vogues Proskauer reagent was added. The nitrate broth contains ...
International Journal of Pharma Tech Research
... The determination of residual solvents in drug substances, excipients or drug products is known to be one of the most difficult and demanding analytical tasks in the pharmaceutical industry. Furthermore, the determination of polar residual solvents in pharmaceutical preparations continues to present ...
... The determination of residual solvents in drug substances, excipients or drug products is known to be one of the most difficult and demanding analytical tasks in the pharmaceutical industry. Furthermore, the determination of polar residual solvents in pharmaceutical preparations continues to present ...
Inhibition of anaerobic digestion by organic priority pollutants
... markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have ...
... markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have ...
2840 GCC 16pp
... The Shielded CO Reactor For the determination of δ18O, the analyte must not contact the ceramic tube which is used to protect against air, and for stability. The pyrolysis takes place in an inert platinum inlay. Due to the catalytic properties of the platinum, the reaction can be performed at 1280 ° ...
... The Shielded CO Reactor For the determination of δ18O, the analyte must not contact the ceramic tube which is used to protect against air, and for stability. The pyrolysis takes place in an inert platinum inlay. Due to the catalytic properties of the platinum, the reaction can be performed at 1280 ° ...
Fulltext: english,
... remaining hydrogen in HisNa+, TrpNa+ and TyrNa+ during the reaction time can be explained as a consequence of two partially positive charges in the zwitterionic form (Figure 1b). In collisions between the sodium cationized amino acid and deuteration reagent, the protonated aamino group and sodium ca ...
... remaining hydrogen in HisNa+, TrpNa+ and TyrNa+ during the reaction time can be explained as a consequence of two partially positive charges in the zwitterionic form (Figure 1b). In collisions between the sodium cationized amino acid and deuteration reagent, the protonated aamino group and sodium ca ...
The source of fermentable carbohydrates influences the in vitro
... The aim of the present study was to determine in vitro, the amount of protein synthesis by faecal microbes, when (1) starch and different sources of purified NSP, or (2) ...
... The aim of the present study was to determine in vitro, the amount of protein synthesis by faecal microbes, when (1) starch and different sources of purified NSP, or (2) ...
Chapter 9 slides
... Gases consist of particles (atoms or molecules) that are relatively far apart. Gas particles move about rapidly. An average O2 molecule moves at a velocity of 980 mi/hr at room temperature. Gas particles have little effect on one another unless they collide. When they collide, they do not stick to ...
... Gases consist of particles (atoms or molecules) that are relatively far apart. Gas particles move about rapidly. An average O2 molecule moves at a velocity of 980 mi/hr at room temperature. Gas particles have little effect on one another unless they collide. When they collide, they do not stick to ...
ARGON Argon
... Sulfates (2.4.13). Dilute 10 mL of solution S to 15 mL with distilled water R. The solution complies with the limit test for sulfates (300 ppm). Ammonium (2.4.1). 50 mg complies with limit test B for ammonium (200 ppm). Prepare the standard using 0.1 mL of ammonium standard solution (100 ppm NH4) R. ...
... Sulfates (2.4.13). Dilute 10 mL of solution S to 15 mL with distilled water R. The solution complies with the limit test for sulfates (300 ppm). Ammonium (2.4.1). 50 mg complies with limit test B for ammonium (200 ppm). Prepare the standard using 0.1 mL of ammonium standard solution (100 ppm NH4) R. ...
Chapter 10: Gases
... You are asked to calculate the volume of a gas sample when only the temperature is changed. You are given the original volume and temperature and the new temperature of the gas sample. According to Charles’s law, temperature and volume are directly related when the pressure and amount of gas are hel ...
... You are asked to calculate the volume of a gas sample when only the temperature is changed. You are given the original volume and temperature and the new temperature of the gas sample. According to Charles’s law, temperature and volume are directly related when the pressure and amount of gas are hel ...
Solutions for Practice Problems
... The small mass of oxygen seems reasonable, given the mole ratio in the balanced chemical equation and the small volume of water vapour that was produced. The answer correctly shows two significant digits. 39. Practice Problem (page 560) One method of producing ammonia gas involves the reaction of am ...
... The small mass of oxygen seems reasonable, given the mole ratio in the balanced chemical equation and the small volume of water vapour that was produced. The answer correctly shows two significant digits. 39. Practice Problem (page 560) One method of producing ammonia gas involves the reaction of am ...
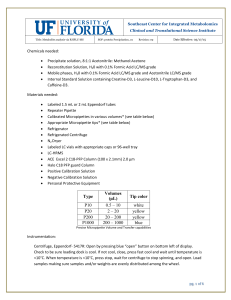
10 white P20 2 - Metabolomics Workbench
... 4- Cool sample in fridge for 30 minutes to further precipitate proteins. 5- Spin at 20,000 rcf for 10 mins at <10° C to create a pellet of proteins. 6- Transfer 750µL of supernatant to new, labeled tube making sure to leave behind protein pellet. 7- Dry liquid sample using Nitrogen gas in Organomati ...
... 4- Cool sample in fridge for 30 minutes to further precipitate proteins. 5- Spin at 20,000 rcf for 10 mins at <10° C to create a pellet of proteins. 6- Transfer 750µL of supernatant to new, labeled tube making sure to leave behind protein pellet. 7- Dry liquid sample using Nitrogen gas in Organomati ...
12 The Gaseous State of Matter Chapter Outline Properties of Gases
... Used in problems involving changes in P, T, and V with a constant amount of gas. ...
... Used in problems involving changes in P, T, and V with a constant amount of gas. ...
Chapter 5
... and 1.3 atm. (For further thought: ammonium bicarbonate (NH4HCO3) has also been used for the same purpose. Think about one advantage and one disadvantage of using NH4HCO3 instead of NaHCO3 for baking.) A) 0.68 L CO2 B) 1.72 L CO2 C) 0.34 L CO2 D) 0.86 L CO2 ...
... and 1.3 atm. (For further thought: ammonium bicarbonate (NH4HCO3) has also been used for the same purpose. Think about one advantage and one disadvantage of using NH4HCO3 instead of NaHCO3 for baking.) A) 0.68 L CO2 B) 1.72 L CO2 C) 0.34 L CO2 D) 0.86 L CO2 ...
Chapter 14: Gases
... Real Gases: - No gases are ideal. - Gas particles have volume (due to size and shape). - Subject to intermolecular forces * Most gases behave like “ideal” gases, except at high pressure and low temps ...
... Real Gases: - No gases are ideal. - Gas particles have volume (due to size and shape). - Subject to intermolecular forces * Most gases behave like “ideal” gases, except at high pressure and low temps ...
5/14/01 - Oklahoma State University
... Problem Solving Resources: This is an interactive feature that can be used to help students develop problem-solving skills. Each tutorial consists of three prototype questions that are randomly sequenced. In the tutorial the student views the first question and is given three choices of how to inter ...
... Problem Solving Resources: This is an interactive feature that can be used to help students develop problem-solving skills. Each tutorial consists of three prototype questions that are randomly sequenced. In the tutorial the student views the first question and is given three choices of how to inter ...
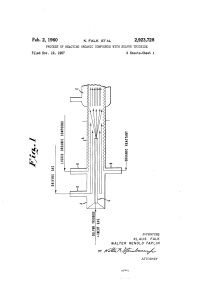
SULFUR TRIOXIDE +INERT GAS
... solid at room temperature. In that event, the organic 0.66 lb. per hour and the air at the rate of 18.18 compound is ?rst melted by a preheater arrangement and ' (S.T.P.) cu. ft. per hour. The reaction mass passing into is fed into the reactor at an elevated temperature in order to keep it in liquid ...
... solid at room temperature. In that event, the organic 0.66 lb. per hour and the air at the rate of 18.18 compound is ?rst melted by a preheater arrangement and ' (S.T.P.) cu. ft. per hour. The reaction mass passing into is fed into the reactor at an elevated temperature in order to keep it in liquid ...
Development of a Photocatalytic Wet Scrubbing - soil
... * To whom correspondence should be addressed. Tel.: +85227666016. Fax: +852-23346389. E-mail: [email protected]. ...
... * To whom correspondence should be addressed. Tel.: +85227666016. Fax: +852-23346389. E-mail: [email protected]. ...
Determination of Fatty Acids and Carbohydrate Monomers in Micro
... MNC 1233) and M . scrofulaceum (MNC 1225 and MNC 1232), isolated from clinical specimens at the Department of Bacteriology, University Hospital, Lund, Sweden. Furthermore, two strains of M. scrofulacmm (MNC 96 and MNC 98, obtained from Dr A. M. Masson, Montreal, Canada) were tested. All strains were ...
... MNC 1233) and M . scrofulaceum (MNC 1225 and MNC 1232), isolated from clinical specimens at the Department of Bacteriology, University Hospital, Lund, Sweden. Furthermore, two strains of M. scrofulacmm (MNC 96 and MNC 98, obtained from Dr A. M. Masson, Montreal, Canada) were tested. All strains were ...
Determination of Fatty Acids and Carbohydrate Monomers in Micro
... MNC 1233) and M . scrofulaceum (MNC 1225 and MNC 1232), isolated from clinical specimens at the Department of Bacteriology, University Hospital, Lund, Sweden. Furthermore, two strains of M. scrofulacmm (MNC 96 and MNC 98, obtained from Dr A. M. Masson, Montreal, Canada) were tested. All strains were ...
... MNC 1233) and M . scrofulaceum (MNC 1225 and MNC 1232), isolated from clinical specimens at the Department of Bacteriology, University Hospital, Lund, Sweden. Furthermore, two strains of M. scrofulacmm (MNC 96 and MNC 98, obtained from Dr A. M. Masson, Montreal, Canada) were tested. All strains were ...
CS gas
The compound 2-chlorobenzalmalononitrile (also called o-chlorobenzylidene malononitrile) (chemical formula: C10H5ClN2), a cyanocarbon, is the defining component of a tear gas commonly referred to as CS gas, which is used as a riot control agent. Exposure causes a burning sensation and tearing of the eyes to the extent that the subject cannot keep their eyes open, and a burning irritation of the nose, mouth and throat mucous membranes causing profuse coughing, mucous nasal discharge, disorientation, and difficulty breathing, partially incapacitating the subject. CS gas is an aerosol of a volatile solvent (a substance that dissolves other active substances and that easily evaporates) and 2-chlorobenzalmalononitrile, which is a solid compound at room temperature. CS gas is generally accepted as being non-lethal. It was discovered by two Americans, Ben Corson and Roger Stoughton, at Middlebury College in 1928, and the chemical's name is derived from the first letters of the scientists' surnames.CS was developed and tested secretly at Porton Down in Wiltshire, England, in the 1950s and 1960s. CS was used first on animals, then subsequently on British Army servicemen volunteers. CS has less effect on animals due to ""under-developed tear-ducts and protection by fur"".