Ch. 5
... behavior of ideal gases. a. The Kinetic Molecular Theory of gases states that : - The particles of a gas are so small compared to the distances between particles that the volume of the individual particles can be assumed to be zero. - The particles of a gas are in constant motion. Gas pressure is cr ...
... behavior of ideal gases. a. The Kinetic Molecular Theory of gases states that : - The particles of a gas are so small compared to the distances between particles that the volume of the individual particles can be assumed to be zero. - The particles of a gas are in constant motion. Gas pressure is cr ...
STUDY OF ZnO, SnO2 AND COMPOUNDS ZTO STRUCTURES
... stable properties under extreme conditions, higher electron mobility compared to its binary counterparts and other interesting properties. These materials is thus ideal for applications from, gas detector, solar cells photocatalysts, lightemitting diodes, field effect transistors and (heterojunction ...
... stable properties under extreme conditions, higher electron mobility compared to its binary counterparts and other interesting properties. These materials is thus ideal for applications from, gas detector, solar cells photocatalysts, lightemitting diodes, field effect transistors and (heterojunction ...
ANSWER KEY Chemistry CPA Final Exam Study Guide Final Exam
... 44. What is the boiling point of: a. substance A? 450 K b. substance B? 250 K ...
... 44. What is the boiling point of: a. substance A? 450 K b. substance B? 250 K ...
Chapter 1 All Things Noncovalent
... A straightforward example is given in structure 1.1. The binding energy of an iodide anion to the doubly charged crown ether is calculated to be ~150 kcal/mol in the gas phase (at the PM5 semi-empirical level of theory). This value is larger than most covalent bond strengths, demonstrating the stren ...
... A straightforward example is given in structure 1.1. The binding energy of an iodide anion to the doubly charged crown ether is calculated to be ~150 kcal/mol in the gas phase (at the PM5 semi-empirical level of theory). This value is larger than most covalent bond strengths, demonstrating the stren ...
elements. annie - Sterling High School
... Who discovered it? Lord Rayleigh and Sir William Ramsay What year was it discovered? 1894 How abundant is it on earth? Argon makes up 0.93% of the earth's atmosphere, Is it a solid, liquid or gas at room temperature? gas Is it metallic or non metallic? nonmetallic What is its melting & boiling point ...
... Who discovered it? Lord Rayleigh and Sir William Ramsay What year was it discovered? 1894 How abundant is it on earth? Argon makes up 0.93% of the earth's atmosphere, Is it a solid, liquid or gas at room temperature? gas Is it metallic or non metallic? nonmetallic What is its melting & boiling point ...
“spontaneous” process: (happens without input of work) Life
... a is the effective equilibrium constant for association of two molecules of gas ...
... a is the effective equilibrium constant for association of two molecules of gas ...
Chapter 5 Gases
... The Ideal Gas Law, PV = nRT, - models the behavior of ideal gases. Other gas laws can be derived from the Ideal Gas Law for either one set of conditions or for two sets of conditions (initial and final conditions). To derive gas laws for two sets of conditions, solve the Ideal Gas Law for R ...
... The Ideal Gas Law, PV = nRT, - models the behavior of ideal gases. Other gas laws can be derived from the Ideal Gas Law for either one set of conditions or for two sets of conditions (initial and final conditions). To derive gas laws for two sets of conditions, solve the Ideal Gas Law for R ...
METHODOLOGY FOR RESIDUAL OXYGEN CALORIMETRY
... To assure complete combustion of a gas, the combustion process is ran at higher oxygen levels than needed to theoretically burn all of the gas given the range and variability of the gas constituent(s) molar percentage content matrix being introduced. This extra amount is called excess oxygen, or bec ...
... To assure complete combustion of a gas, the combustion process is ran at higher oxygen levels than needed to theoretically burn all of the gas given the range and variability of the gas constituent(s) molar percentage content matrix being introduced. This extra amount is called excess oxygen, or bec ...
presentation on power generation from biogas in 2
... H2S gets absorbed in water, The gas from the bottom of the Scrubber enters the packed Column while liquid is collected in the Tank for recalculation. In the II stage of scrubbing, gas is scrubbed with caustic solution in a packed Column. The Column is provided with ceramic rings to have enhanced sur ...
... H2S gets absorbed in water, The gas from the bottom of the Scrubber enters the packed Column while liquid is collected in the Tank for recalculation. In the II stage of scrubbing, gas is scrubbed with caustic solution in a packed Column. The Column is provided with ceramic rings to have enhanced sur ...
Suggest a reason for the large difference in the boiling points of
... 2 PCl3 fumes in moist air. 3 conc. H2SO4 is a strong dehydrating agent. 4 of noble gases , only xenon is known to form real chemical compound 2 Explain mechanism of dehydration of alcohol for formation of 1) ethers 2)alkene 3 Suggest a reason for the large difference in the boiling points of butanol ...
... 2 PCl3 fumes in moist air. 3 conc. H2SO4 is a strong dehydrating agent. 4 of noble gases , only xenon is known to form real chemical compound 2 Explain mechanism of dehydration of alcohol for formation of 1) ethers 2)alkene 3 Suggest a reason for the large difference in the boiling points of butanol ...
Exp`t 70
... • To the distillate add 0.3 mL cold 3 M sodium hydroxide solution to neutralize sulfurous acid, mix well, draw off the aqueous layer, and dry the ORGANIC product over anhydrous calcium chloride pellets, adding the drying agent in small quantities until it no longer clumps together. Keep the vial col ...
... • To the distillate add 0.3 mL cold 3 M sodium hydroxide solution to neutralize sulfurous acid, mix well, draw off the aqueous layer, and dry the ORGANIC product over anhydrous calcium chloride pellets, adding the drying agent in small quantities until it no longer clumps together. Keep the vial col ...
AP gas notes 2010
... 4. Collisions of gas molecules are ELASTIC. NO KINETIC ENERGY IS LOST! 5. The Average Kinetic Energy of gas molecules is directly proportional to KELVIN temperatures At a given temperature, the gas particles of Sample A have the same KE as the gas particles of Sample B. ...
... 4. Collisions of gas molecules are ELASTIC. NO KINETIC ENERGY IS LOST! 5. The Average Kinetic Energy of gas molecules is directly proportional to KELVIN temperatures At a given temperature, the gas particles of Sample A have the same KE as the gas particles of Sample B. ...
Candidates should check the question paper to
... (a) (i) On the grid provided, plot a graph of solubility of A and B ( y-axis) against temperature. ...
... (a) (i) On the grid provided, plot a graph of solubility of A and B ( y-axis) against temperature. ...
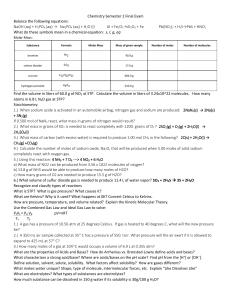
Chemistry Semester 2 Final Exam Chemistry Semester 2 Final Exam
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
... 1.) A gas has a pressure of 10.56 atm at 25 degrees Celcius. If gas is heated to 40 degrees C, what will the new pressure be? 10.0 atm 2.) A 350 mL air sample collected at 35 C has a pressure of 550. torr. What pressure will the air exert if it is allowed to expand to 425 mL at 57 C? 485 torr 3.) ...
Chapter 3 Part 2 Review
... With your partner, solve the problem and show all your work. Raise your hand when you are done. First group with the correct answer gets the point and a chance at the bonus point. ...
... With your partner, solve the problem and show all your work. Raise your hand when you are done. First group with the correct answer gets the point and a chance at the bonus point. ...
Lab Stuff - WW-P K
... What types of substances are removed from mixtures using filtration? Adsorption? Distillation? Think about the foul water lab! ...
... What types of substances are removed from mixtures using filtration? Adsorption? Distillation? Think about the foul water lab! ...
Lab Stuff:
... What types of substances are removed from mixtures using filtration? Adsorption? Distillation? Think about the foul water lab! ...
... What types of substances are removed from mixtures using filtration? Adsorption? Distillation? Think about the foul water lab! ...
Lab Stuff
... What types of substances are removed from mixtures using filtration? Adsorption? Distillation? Think about the foul water lab! ...
... What types of substances are removed from mixtures using filtration? Adsorption? Distillation? Think about the foul water lab! ...
the nakuru district sec. schools trial examinations - 2015
... 14 Red-hot iron reacts with steam to give tri-iron tetroxide and hydrogen gas. The reaction is reversible 3Fe(s) + 4H2O(s) Fe304(s) + 4H2(g) (a) Define dynamic equilibrium (1 mark) Although the reaction appears to have stopped by attaining a state of balance, both forward and backward reactions ar ...
... 14 Red-hot iron reacts with steam to give tri-iron tetroxide and hydrogen gas. The reaction is reversible 3Fe(s) + 4H2O(s) Fe304(s) + 4H2(g) (a) Define dynamic equilibrium (1 mark) Although the reaction appears to have stopped by attaining a state of balance, both forward and backward reactions ar ...
Chemistry 2nd Semester Final Review
... There are only two types of pure substances: elements and compounds A mixture is anything that is not a single element or a single compound, but a mixture of more than one element or compound Mixtures can be separated through physical means. For example, if you want to remove salt from water, perfor ...
... There are only two types of pure substances: elements and compounds A mixture is anything that is not a single element or a single compound, but a mixture of more than one element or compound Mixtures can be separated through physical means. For example, if you want to remove salt from water, perfor ...
Chemistry- The Gas Phase
... One way H2 gas can be produced commercially is to react a strong acid with a metal: 2HCl(aq) + Zn(s) → H2(g) + ZnCl2(aq) If 512 g of HCl are reacted with excess Zn at T=24 oC and P=0.95 atm, what volume of H2 gas will be produced? ...
... One way H2 gas can be produced commercially is to react a strong acid with a metal: 2HCl(aq) + Zn(s) → H2(g) + ZnCl2(aq) If 512 g of HCl are reacted with excess Zn at T=24 oC and P=0.95 atm, what volume of H2 gas will be produced? ...
File - Garbally Chemistry
... The Alkanes Formerly known as the ‘paraffins’ (from the Latin meaning ‘lacking affinity’) the alkanes are a group of hydrocarbons which conform to the general formula CnH2n+2, where n is greater than or equal to 1. They are saturated compounds. They consist of carbon atoms joined by four single cova ...
... The Alkanes Formerly known as the ‘paraffins’ (from the Latin meaning ‘lacking affinity’) the alkanes are a group of hydrocarbons which conform to the general formula CnH2n+2, where n is greater than or equal to 1. They are saturated compounds. They consist of carbon atoms joined by four single cova ...
0191 271 0222 Nitrogen (Oxygen Free)
... Ensure all national and local regulations are observed. R-phrase(s) Substance/preparation : R00 There are no known health hazards. Components prepared by : Dixons of Westerhope Limited. This safety data sheet has been established in accordance with the applicable European Directives and applies to a ...
... Ensure all national and local regulations are observed. R-phrase(s) Substance/preparation : R00 There are no known health hazards. Components prepared by : Dixons of Westerhope Limited. This safety data sheet has been established in accordance with the applicable European Directives and applies to a ...
Test 4 - UTC.edu
... Chemistry 121 Test 4 Spring 2007 You have 75 minutes to complete this 100 point test. Please mark each answer clearly and show all work. You may use a simple scientific calculator. NO GAPHING CALCULATORS. I. Fill in the blank 1. (1 pt) The weakest intermolecular forces are called ___________________ ...
... Chemistry 121 Test 4 Spring 2007 You have 75 minutes to complete this 100 point test. Please mark each answer clearly and show all work. You may use a simple scientific calculator. NO GAPHING CALCULATORS. I. Fill in the blank 1. (1 pt) The weakest intermolecular forces are called ___________________ ...
CS gas
The compound 2-chlorobenzalmalononitrile (also called o-chlorobenzylidene malononitrile) (chemical formula: C10H5ClN2), a cyanocarbon, is the defining component of a tear gas commonly referred to as CS gas, which is used as a riot control agent. Exposure causes a burning sensation and tearing of the eyes to the extent that the subject cannot keep their eyes open, and a burning irritation of the nose, mouth and throat mucous membranes causing profuse coughing, mucous nasal discharge, disorientation, and difficulty breathing, partially incapacitating the subject. CS gas is an aerosol of a volatile solvent (a substance that dissolves other active substances and that easily evaporates) and 2-chlorobenzalmalononitrile, which is a solid compound at room temperature. CS gas is generally accepted as being non-lethal. It was discovered by two Americans, Ben Corson and Roger Stoughton, at Middlebury College in 1928, and the chemical's name is derived from the first letters of the scientists' surnames.CS was developed and tested secretly at Porton Down in Wiltshire, England, in the 1950s and 1960s. CS was used first on animals, then subsequently on British Army servicemen volunteers. CS has less effect on animals due to ""under-developed tear-ducts and protection by fur"".