Lecture content: How do amino acids differ from carbohydrates and
... 1. How is the NH3-group separated from the carbon ”skeleton” of the amino acid? 2. How is ammonia converted to urea? 3. What happens with the carbon ”skeleton”? ...
... 1. How is the NH3-group separated from the carbon ”skeleton” of the amino acid? 2. How is ammonia converted to urea? 3. What happens with the carbon ”skeleton”? ...
ENERGY
... CO2 is more stable ( less complex) Reactants are glucose and oxygen Products are carbon dioxide and water Energy is now in the form of ATP Organic compounds are the main fuel source ...
... CO2 is more stable ( less complex) Reactants are glucose and oxygen Products are carbon dioxide and water Energy is now in the form of ATP Organic compounds are the main fuel source ...
Formation of pyruvic acid (P
... 1- TCA cycle (tricarboxylic acid cycle), also known as the citric acid cycle or the Krebs’ cycle, is the major energy production pathways in the body. *The cycle occurs in the mitochondria. 2- It is a cyclic process. 3-The cycle involves a sequence of compounds inter-related by oxidationreduction an ...
... 1- TCA cycle (tricarboxylic acid cycle), also known as the citric acid cycle or the Krebs’ cycle, is the major energy production pathways in the body. *The cycle occurs in the mitochondria. 2- It is a cyclic process. 3-The cycle involves a sequence of compounds inter-related by oxidationreduction an ...
Bio 20 7.4 - Stirling School
... 2 Components to VO2 Max Exercise and Genetics. When an individual increases there levels of exercise, their VO2 max will generally increase as well. Genetic composition plays a part as well. This is why some people are elite marathon runners and some are not. VO2 max also decreases with a ...
... 2 Components to VO2 Max Exercise and Genetics. When an individual increases there levels of exercise, their VO2 max will generally increase as well. Genetic composition plays a part as well. This is why some people are elite marathon runners and some are not. VO2 max also decreases with a ...
2. tissue - specific metabolism - cmb
... 2. The heart is a completely aerobic tissue, whereas skeletal muscle can function anaerobically for limited periods. Mitochondria are much more densely packed in heart than in other cells, making up nearly half the volume of a heart cell. 3. The heart contains negligible energy reserves as glycogen ...
... 2. The heart is a completely aerobic tissue, whereas skeletal muscle can function anaerobically for limited periods. Mitochondria are much more densely packed in heart than in other cells, making up nearly half the volume of a heart cell. 3. The heart contains negligible energy reserves as glycogen ...
introacidbase
... In a titration if we add base to the acid: HA + OH- - H2O + AFor every mole of HA titrated, we form a mole of ASo, if we add enough OH- to use up half the HA (it is half-titrated) we end up with equimolar HA and ALooking at the equation: ...
... In a titration if we add base to the acid: HA + OH- - H2O + AFor every mole of HA titrated, we form a mole of ASo, if we add enough OH- to use up half the HA (it is half-titrated) we end up with equimolar HA and ALooking at the equation: ...
Brain Needs in Different Metabolic states
... required to sustain respiration in all cells. Is expressed by renal tubular cells and small intestinal epithelial cells that transport glucose, liver cells and pancreatic beta cells. All three monosaccharides are . transported from the intestinal mucosal cell into the portal circulation by GLUT2 ...
... required to sustain respiration in all cells. Is expressed by renal tubular cells and small intestinal epithelial cells that transport glucose, liver cells and pancreatic beta cells. All three monosaccharides are . transported from the intestinal mucosal cell into the portal circulation by GLUT2 ...
Chapter 5 - Scranton Prep Biology
... hydro- = water; -lyse = break (hydrolysis:breaking chemicalbonds by adding water) macro- = large (macromolecule: alarge molecule) meros- - part (polymer:a chain made from smaller organic molecules) mono- = single; -sacchar= sugar (monosaccharide: simplest type of sugar) poly- = rnany (polysaccharide ...
... hydro- = water; -lyse = break (hydrolysis:breaking chemicalbonds by adding water) macro- = large (macromolecule: alarge molecule) meros- - part (polymer:a chain made from smaller organic molecules) mono- = single; -sacchar= sugar (monosaccharide: simplest type of sugar) poly- = rnany (polysaccharide ...
(PDF, Unknown)
... supplementation when otherwise deficient (vegans, vegetarians with low dairy intake) or during prolonged endurance exercise. Anecdotally reported to reduce sugar cravings. Research shows glutamine builds muscle when tested on those suffering from physical trauma such as burns or muscular wounds (kni ...
... supplementation when otherwise deficient (vegans, vegetarians with low dairy intake) or during prolonged endurance exercise. Anecdotally reported to reduce sugar cravings. Research shows glutamine builds muscle when tested on those suffering from physical trauma such as burns or muscular wounds (kni ...
Problem Set 5 (Due February 25th) 1. Show how glucose can be
... d. How was enzyme activity monitored? Monitoring the reduction of NAD+ to NADH spectrophotometrically – I noticed that the experimental section refers to another paper, so I apologize if this gave you a headache. e. Figure 5 has a lot of important information. i. What does this figure tell us about ...
... d. How was enzyme activity monitored? Monitoring the reduction of NAD+ to NADH spectrophotometrically – I noticed that the experimental section refers to another paper, so I apologize if this gave you a headache. e. Figure 5 has a lot of important information. i. What does this figure tell us about ...
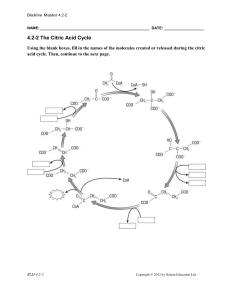
Blackline Master 4.2-2 NAME: DATE: 4.2
... ________________enters the cycle and then combines with ________________ to make the six-carbon compound ________________. During the eight steps of the citric cycle, ________________ undergoes a number of reactions, releasing _______ and ______ in a number of steps. ________________ is eventually c ...
... ________________enters the cycle and then combines with ________________ to make the six-carbon compound ________________. During the eight steps of the citric cycle, ________________ undergoes a number of reactions, releasing _______ and ______ in a number of steps. ________________ is eventually c ...
Describe and discuss the process of chemiosmosis in eukaryotic
... 1. Cellular Respiration is the cornerstone of metabolism. A. Trace the pathway of electrons from glucose through the entire process of aerobic cellular respiration and describe all significant events in which energy is transferred between molecules. (3 pt maximum) __Redox: Energy is derived from el ...
... 1. Cellular Respiration is the cornerstone of metabolism. A. Trace the pathway of electrons from glucose through the entire process of aerobic cellular respiration and describe all significant events in which energy is transferred between molecules. (3 pt maximum) __Redox: Energy is derived from el ...
Page 1 Introduction to Biochemistry
... as shown by glucose. 11. Disaccharides are formed by joining two hexose units (as shown by sucrose, maltose and lactose). The bond formed between monosaccharides is a glycosidic bond. 12. Glucose exists as two isomers (alpha and beta) and glucose forms different polymers; starch (amylose and amylope ...
... as shown by glucose. 11. Disaccharides are formed by joining two hexose units (as shown by sucrose, maltose and lactose). The bond formed between monosaccharides is a glycosidic bond. 12. Glucose exists as two isomers (alpha and beta) and glucose forms different polymers; starch (amylose and amylope ...
Cellular Respiration 1. To perform cell work, cells require energy. a
... low energy products. Some of the released energy is used to do work; the rest is lost as heat. Specifically, 40% is converted to ATP while 60% of the energy from glucose is lost as heat. Some of that heat is used to maintain our high body temperature (37C). d. Cells harvest the chemical energy stor ...
... low energy products. Some of the released energy is used to do work; the rest is lost as heat. Specifically, 40% is converted to ATP while 60% of the energy from glucose is lost as heat. Some of that heat is used to maintain our high body temperature (37C). d. Cells harvest the chemical energy stor ...
(a) (b)
... (b) Second law of thermodynamics: Every energy transfer or transformation increases the disorder (entropy) of the universe. For example, disorder is added to the cheetah’s surroundings in the form of heat and the small molecules that are the by-products of metabolism. ...
... (b) Second law of thermodynamics: Every energy transfer or transformation increases the disorder (entropy) of the universe. For example, disorder is added to the cheetah’s surroundings in the form of heat and the small molecules that are the by-products of metabolism. ...
Describe
... Autotrophs make their own food by using energy from sunlight or inorganic substances to build organic compounds. Many autotrophs make food by the process of photosynthesis. •Breaking Down Food for Energy Energy from sunlight flows through living systems, from autotrophs to heterotrophs. Heterotrophs ...
... Autotrophs make their own food by using energy from sunlight or inorganic substances to build organic compounds. Many autotrophs make food by the process of photosynthesis. •Breaking Down Food for Energy Energy from sunlight flows through living systems, from autotrophs to heterotrophs. Heterotrophs ...
Lecture #10 – 9/26 – Dr. Hirsh
... Form ATP by “burning” NADH through respiration (oxidative phosphorylation) This is a redox reaction with NADH as an intermediate; reduced A (AH2) has a higher energy level than reduced B (BH2) – therefore we have a source of energy for proton pumping. We need to get energy out of he process in small ...
... Form ATP by “burning” NADH through respiration (oxidative phosphorylation) This is a redox reaction with NADH as an intermediate; reduced A (AH2) has a higher energy level than reduced B (BH2) – therefore we have a source of energy for proton pumping. We need to get energy out of he process in small ...
LT AP BIO
... C6H12O6 + 6O2 ---> 6CO2 + 6H2O + Energy (ATP + heat) Products of photosynthesis are the reactants of the cellular respiration ...
... C6H12O6 + 6O2 ---> 6CO2 + 6H2O + Energy (ATP + heat) Products of photosynthesis are the reactants of the cellular respiration ...
16 Proteins/Vitamins
... High protein – low carb diets (Atkins, Zone, Protein Power…) Result in quick weight loss because eliminating carbohydrates results in loss of body fluids – but, according to the American Heart Association, are not effective long term: impede fat metabolism, generally substitute carbohydrates with f ...
... High protein – low carb diets (Atkins, Zone, Protein Power…) Result in quick weight loss because eliminating carbohydrates results in loss of body fluids – but, according to the American Heart Association, are not effective long term: impede fat metabolism, generally substitute carbohydrates with f ...
Bio Honors Review Packet
... 4. If the pH changes from a pH of 3 to a pH of 2, the concentration of hydrogen ions increases by 100x. 5. Hydrolysis reactions involve the removal of water to join two simpler molecules to form a more complex molecule. 6. The beta structure of a protein is a pleated sheet type of kind of structure ...
... 4. If the pH changes from a pH of 3 to a pH of 2, the concentration of hydrogen ions increases by 100x. 5. Hydrolysis reactions involve the removal of water to join two simpler molecules to form a more complex molecule. 6. The beta structure of a protein is a pleated sheet type of kind of structure ...
Table S1.
... Regulates cholesterol and fatty acids by activating Cyp7a , the rate limiting enzyme in the conversion of cholesterol to bile acid. Peroxisome proliferator-activated receptor Role in promoting hepatic fatty acid oxidation and ...
... Regulates cholesterol and fatty acids by activating Cyp7a , the rate limiting enzyme in the conversion of cholesterol to bile acid. Peroxisome proliferator-activated receptor Role in promoting hepatic fatty acid oxidation and ...
Bioloical Oxidation - Home
... Because ATP has a high energy bonds. It can serve as a link between energy yielding processes or exergonic reactions •e.g. catabolism of glucose and fatty acids and energy requiring process or enderogenic reactions (e.g. anabolic pathways) A) catabolic reaction give energy , which can be stored in t ...
... Because ATP has a high energy bonds. It can serve as a link between energy yielding processes or exergonic reactions •e.g. catabolism of glucose and fatty acids and energy requiring process or enderogenic reactions (e.g. anabolic pathways) A) catabolic reaction give energy , which can be stored in t ...
Chapter 25
... Chapter 25 Metabolism and Nutrition • The food we eat is our only source of energy for performing biological work. • There are three major metabolic destinations for the principle nutrients. They will be used for energy for active processes, synthesized into structural or functional molecules, or sy ...
... Chapter 25 Metabolism and Nutrition • The food we eat is our only source of energy for performing biological work. • There are three major metabolic destinations for the principle nutrients. They will be used for energy for active processes, synthesized into structural or functional molecules, or sy ...
Ch. 7.4: Cellular Respiration
... What: Making ATP w/out O2 (using glycolysis) Context: Working muscles need an ongoing ATP supply; faster than O2 can be supplied for respiration. Yield: 2 ATPs for each glucose (but regular respiration is ...
... What: Making ATP w/out O2 (using glycolysis) Context: Working muscles need an ongoing ATP supply; faster than O2 can be supplied for respiration. Yield: 2 ATPs for each glucose (but regular respiration is ...
Basal metabolic rate

Basal metabolic rate (BMR) is the minimal rate of energy expenditure per unit time by endothermic animals at rest. (McNab, B. K. 1997). On the Utility of Uniformity in the Definition of Basal Rate of Metabolism. Physiol. Zool. Vol.70; Metabolism refers to the processes that the body needs to function. Basal Metabolic Rate is the amount of energy expressed in calories that a person needs to keep the body functioning at rest. Some of those processes are breathing, blood circulation, controlling body temperature, cell growth, brain and nerve function, and contraction of muscles. Basal metabolic rate (BMR) affects the rate that a person burns calories and ultimately whether you maintain, gain, or lose weight. Your basal metabolic rate accounts for about 60 to 75% of the calories you burn every day. It is influenced by several factors.