0c5168dab2ecd61778b5bb175973dab5 UNPDF
... monomers together in a certain sequence/order they have ______________________ a. The process of “putting monomers together” is called b. What is lost during the process ? c. What kind of bond is formed generally? Specifically between amino acids of a ...
... monomers together in a certain sequence/order they have ______________________ a. The process of “putting monomers together” is called b. What is lost during the process ? c. What kind of bond is formed generally? Specifically between amino acids of a ...
topic 4 - biochemistry - part 1 - organic compounds
... **Generally: The order in which the amino acids are linked together, determines the characteristics of the protein molecule. **Based on this sequence, the protein chains twist, turn, & bend into specific 3-D shapes. -The shape of a protein molecule is its: _______________________________________ -T ...
... **Generally: The order in which the amino acids are linked together, determines the characteristics of the protein molecule. **Based on this sequence, the protein chains twist, turn, & bend into specific 3-D shapes. -The shape of a protein molecule is its: _______________________________________ -T ...
Chapter-1-Structure-and-Variety-of-Protein
... • Tertiary structure – polypeptides become further linked by various cross-connections. • The types of connections formed at this stage are important, they determine final structure and allow it to carry out a specific ...
... • Tertiary structure – polypeptides become further linked by various cross-connections. • The types of connections formed at this stage are important, they determine final structure and allow it to carry out a specific ...
1.0 amino acids as units of protein structure
... Cells contain thousands of different proteins. A major problem for protein chemists is to purify a chosen protein so that they can study its specific properties in the absence of other proteins. Because the biological function of a protein depends on its native structure, techniques employed in prot ...
... Cells contain thousands of different proteins. A major problem for protein chemists is to purify a chosen protein so that they can study its specific properties in the absence of other proteins. Because the biological function of a protein depends on its native structure, techniques employed in prot ...
structural
... protein is stabilized by all types of bonds between the side chains… ionic between charged AA’s, Hydrogen bonds between polar AA’s, van der Waals forces, and even covalent bonds between sulfurs. ...
... protein is stabilized by all types of bonds between the side chains… ionic between charged AA’s, Hydrogen bonds between polar AA’s, van der Waals forces, and even covalent bonds between sulfurs. ...
Protein Stability Protein Folding
... • The proteins found in thermophilic species are much more stable than their mesophilic counterparts (although this corresponds to only 3 - 8 kcal/mol of free energy). • However, the overall three-dimensional structures will be essentially the same for both thermophilic and mesophilic proteins. • It ...
... • The proteins found in thermophilic species are much more stable than their mesophilic counterparts (although this corresponds to only 3 - 8 kcal/mol of free energy). • However, the overall three-dimensional structures will be essentially the same for both thermophilic and mesophilic proteins. • It ...
Newsletter 9th Edition – Mar 8, 2017
... can then use these building blocks to manufacture some 50,000 different body proteins – each of which has a specific structure (and function) based upon its arrangement of amino acids. As long as your body has all the necessary “raw materials” in the form of the amino acid building blocks, it can ma ...
... can then use these building blocks to manufacture some 50,000 different body proteins – each of which has a specific structure (and function) based upon its arrangement of amino acids. As long as your body has all the necessary “raw materials” in the form of the amino acid building blocks, it can ma ...
HSPIR: a manually annotated heat shock protein information resource
... HSPIR incorporates four different search features. Basic keyword search allows finding of HSPs based on their names (includes gene names, standardized and synonymous names), families, identifiers (HSPIR and external) and classifications. The advanced search is our key feature, which narrows the sear ...
... HSPIR incorporates four different search features. Basic keyword search allows finding of HSPs based on their names (includes gene names, standardized and synonymous names), families, identifiers (HSPIR and external) and classifications. The advanced search is our key feature, which narrows the sear ...
26490 Demonstrate knowledge of the structure, properties
... chemical structure and properties of amino acids; describe the structure and functions of peptides and proteins; and explain the physical and chemical properties of proteins. ...
... chemical structure and properties of amino acids; describe the structure and functions of peptides and proteins; and explain the physical and chemical properties of proteins. ...
Fundamentals of Cell Biology
... – Virtually all protein synthesis is centralized in the cytosol for eukaryotic cells, and many of these proteins are targeted to specific cellular locations by signal sequences. – Proteins that enter and leave the nucleus are maintained in a functional shape at all times. – Proteins enter the peroxi ...
... – Virtually all protein synthesis is centralized in the cytosol for eukaryotic cells, and many of these proteins are targeted to specific cellular locations by signal sequences. – Proteins that enter and leave the nucleus are maintained in a functional shape at all times. – Proteins enter the peroxi ...
Amino Acids and Proteins
... structure include the alpha helix, pleated sheet and collagen. The interaction of side groups to form the cross-links of tertiary structure is discussed. The breakdown in the secondary and tertiary structural levels is described as part of a discussion on denaturation of proteins. The discussion inc ...
... structure include the alpha helix, pleated sheet and collagen. The interaction of side groups to form the cross-links of tertiary structure is discussed. The breakdown in the secondary and tertiary structural levels is described as part of a discussion on denaturation of proteins. The discussion inc ...
sc-PDB: an annotated database of druggable binding sites from the
... [1] Kellenberger, E., Muller, P., Schalon, C., Bret, G., Foata, N. and Rognan, D. (2006). sc-PDB: an Annotated Database of Druggable Binding Sites from the Protein Data Bank J. chem. Inf. Model. 46, 717-727. [2] Surgand, J.-S.; Rodrigo, J.; Kellenberger, E. and Rognan, D. (2006). A chemogenomic anal ...
... [1] Kellenberger, E., Muller, P., Schalon, C., Bret, G., Foata, N. and Rognan, D. (2006). sc-PDB: an Annotated Database of Druggable Binding Sites from the Protein Data Bank J. chem. Inf. Model. 46, 717-727. [2] Surgand, J.-S.; Rodrigo, J.; Kellenberger, E. and Rognan, D. (2006). A chemogenomic anal ...
PDF(343KB)
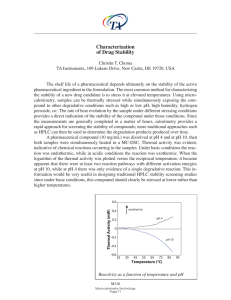
... pharmaceutical ingredient in the formulation. The most common method for characterizing the stability of a new drug candidate is to stress it at elevated temperatures. Using microcalorimetry, samples can be thermally stressed while simultaneously exposing the compound to other degradative conditions ...
... pharmaceutical ingredient in the formulation. The most common method for characterizing the stability of a new drug candidate is to stress it at elevated temperatures. Using microcalorimetry, samples can be thermally stressed while simultaneously exposing the compound to other degradative conditions ...
Protein Unit Study Guide/Review Sheets
... What element(s) ALWAYS comprise proteins? C, H, O, N Are proteins organic? YES What element(s) MAY be present in proteins? S What is the name of the monomer of proteins? AMINO ACID What type of bond links amino acids together? PEPTIDE BOND What functional groups is shared between ALL amino acids (gi ...
... What element(s) ALWAYS comprise proteins? C, H, O, N Are proteins organic? YES What element(s) MAY be present in proteins? S What is the name of the monomer of proteins? AMINO ACID What type of bond links amino acids together? PEPTIDE BOND What functional groups is shared between ALL amino acids (gi ...
Endo-1-06-99_1-20-99
... Transport: Need an intact vascular system or hormones can’t get to where they’re going Solubility (peptides are water-soluble, steroids are not water-soluble) Steroids have to be altered by transport proteins to be water-soluble (also see this with lipids and cholesterol) Lipid transport: Uses the p ...
... Transport: Need an intact vascular system or hormones can’t get to where they’re going Solubility (peptides are water-soluble, steroids are not water-soluble) Steroids have to be altered by transport proteins to be water-soluble (also see this with lipids and cholesterol) Lipid transport: Uses the p ...
DNA-templated CMV Viral Coat Protein Assemble Into Nanotubes
... forward primer: 5’-CCC TTA TGT TAC GTC CTG-3’ and the reverse primer: 5’-TGG TGT AGA GCA TTA CGC-3’. The amplification was carried out using the following thermal cycling profile: 94°C for 3 min, followed by 30 cycles of amplification (94°C for 30 s, 55°C for 30 s, 72°C for 30 s). Finally, the react ...
... forward primer: 5’-CCC TTA TGT TAC GTC CTG-3’ and the reverse primer: 5’-TGG TGT AGA GCA TTA CGC-3’. The amplification was carried out using the following thermal cycling profile: 94°C for 3 min, followed by 30 cycles of amplification (94°C for 30 s, 55°C for 30 s, 72°C for 30 s). Finally, the react ...
Some General Information on CD of Proteins
... CD bands from individual residues may be positive or negative and may vary widely in intensity, so it is often difficult to separate out the contributions of individual aromatic residues. The signals may also cancel each other out, so no near UV CD signal does not necessarily mean no tertiary struct ...
... CD bands from individual residues may be positive or negative and may vary widely in intensity, so it is often difficult to separate out the contributions of individual aromatic residues. The signals may also cancel each other out, so no near UV CD signal does not necessarily mean no tertiary struct ...
19-9-ET-V1-S1__preci..
... molecule from the protein. Therefore the ionic interactions between water molecules and protein are reduced and as result hydrophobic interactions dominate. The hydrophobic amino acid patches present in all the proteins attract each other and forms aggregates. These aggregates are nothing but the pr ...
... molecule from the protein. Therefore the ionic interactions between water molecules and protein are reduced and as result hydrophobic interactions dominate. The hydrophobic amino acid patches present in all the proteins attract each other and forms aggregates. These aggregates are nothing but the pr ...
proteins - LSU Macro Sites
... protein dictate its structure? OR How can we predict the structure of a protein from its sequence? ---Levanthal’s Paradox: if each amino acid can be in the alpha helix, beta sheet, or random coil configuration, then there are 3100 different possible conformational forms of this protein If each possi ...
... protein dictate its structure? OR How can we predict the structure of a protein from its sequence? ---Levanthal’s Paradox: if each amino acid can be in the alpha helix, beta sheet, or random coil configuration, then there are 3100 different possible conformational forms of this protein If each possi ...
Protein Folding Problem
... The initial stages of folding must be nearly random, but if the entire process was a random search it would require too much time. Consider a 100 residue protein. If each residue is considered to have just 3 possible conformations the total number of conformations of the protein is 3100. Conformatio ...
... The initial stages of folding must be nearly random, but if the entire process was a random search it would require too much time. Consider a 100 residue protein. If each residue is considered to have just 3 possible conformations the total number of conformations of the protein is 3100. Conformatio ...
Thermo Scientific Top Vision Low Melting Point Agarose
... this product for internal research and development purposes. No other license is granted to the buyer whether expressly, by implication, by estoppel or otherwise. In particular, the purchase of the product does not include or carry any right or license to use, develop, or otherwise exploit this prod ...
... this product for internal research and development purposes. No other license is granted to the buyer whether expressly, by implication, by estoppel or otherwise. In particular, the purchase of the product does not include or carry any right or license to use, develop, or otherwise exploit this prod ...
bio-of-cells-essay-2 156 kb bio-of-cells-essay
... conformational change in shape. Then the carrier releases the solute on the other side of the membrane, and returns to its original state. An example of passive transmembrane transport by carrier proteins is by the plasma membrane protein GLUT 1, which is used to facilitate the uptake glucose into c ...
... conformational change in shape. Then the carrier releases the solute on the other side of the membrane, and returns to its original state. An example of passive transmembrane transport by carrier proteins is by the plasma membrane protein GLUT 1, which is used to facilitate the uptake glucose into c ...
Determination of Protein Concentration
... may absorb UV light or modify the molar absorptivities of tyrosine and tryptophan, and thus the UV detection is highly sensitive to pH and ionic strength at which measurement is taken. Many other cellular components, and particularly nucleic acids, also absorb UV light. The ratio of A 280 /A 260 is ...
... may absorb UV light or modify the molar absorptivities of tyrosine and tryptophan, and thus the UV detection is highly sensitive to pH and ionic strength at which measurement is taken. Many other cellular components, and particularly nucleic acids, also absorb UV light. The ratio of A 280 /A 260 is ...