Bioenergetics
... Increased muscle mitochondrial content More effective sparing of CHO for use by the central nervous system Blunted drop in intracellular pH during a longterm aerobic endurance event ...
... Increased muscle mitochondrial content More effective sparing of CHO for use by the central nervous system Blunted drop in intracellular pH during a longterm aerobic endurance event ...
Introduction to Metabolism
... ORGANISMS are energy TRANSFORMERS! Spontaneous processes occur without energy input; they can happen quickly or slowly For a process to occur without energy input, it must increase the entropy of the universe ...
... ORGANISMS are energy TRANSFORMERS! Spontaneous processes occur without energy input; they can happen quickly or slowly For a process to occur without energy input, it must increase the entropy of the universe ...
Ch 8 - Bartlett High School
... • All of an organisms chemical processes 2. What are the different types of metabolism? • Catabolism – releases energy by breaking down complex molecules • Anabolism – use energy to build up complex molecules • Catabolic rxns – hydrolysis – break bonds • Anabolic rxns – dehydration – form bonds 3. H ...
... • All of an organisms chemical processes 2. What are the different types of metabolism? • Catabolism – releases energy by breaking down complex molecules • Anabolism – use energy to build up complex molecules • Catabolic rxns – hydrolysis – break bonds • Anabolic rxns – dehydration – form bonds 3. H ...
Biology 181: Study Guide
... If glucose provides the ultimate source of energy for cells, why do they transfer that energy to other molecules like ADP -> ATP? or NAD+ -> NADH? Compare substrate-level phosphorylation to oxidative phosphorylation. Which require enzymes? what are the oxidizing agents? where do these processes occu ...
... If glucose provides the ultimate source of energy for cells, why do they transfer that energy to other molecules like ADP -> ATP? or NAD+ -> NADH? Compare substrate-level phosphorylation to oxidative phosphorylation. Which require enzymes? what are the oxidizing agents? where do these processes occu ...
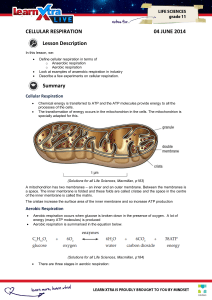
CELLULAR RESPIRATION 04 JUNE 2014 Lesson Description
... Oxidative phosphorylation: takes the energy from the energy-rich hydrogens to make ATP. The energy depleted hydrogens combine with oxygen to make water. This is either breathed out as water vapour or excreted via the kidneys. ...
... Oxidative phosphorylation: takes the energy from the energy-rich hydrogens to make ATP. The energy depleted hydrogens combine with oxygen to make water. This is either breathed out as water vapour or excreted via the kidneys. ...
Nerve activates contraction
... synthesis via the proton gradient and ATP synthase. This occurs primarily in the presence of oxygen. Chemiosmosisthe phosphorylation of ADP to ATP occurring when protons that are following a concentration gradient contact ATP synthase. ...
... synthesis via the proton gradient and ATP synthase. This occurs primarily in the presence of oxygen. Chemiosmosisthe phosphorylation of ADP to ATP occurring when protons that are following a concentration gradient contact ATP synthase. ...
Enzymes
... Enzymes ●Some chemical reactions that make life possible are too slow or have activation energies that are too high to make them practical for living tissue. ●These chemical reactions are made possible by catalysts (biological catalysts are Enzymes). How: Lower Activation energy ...
... Enzymes ●Some chemical reactions that make life possible are too slow or have activation energies that are too high to make them practical for living tissue. ●These chemical reactions are made possible by catalysts (biological catalysts are Enzymes). How: Lower Activation energy ...
Why ATP?
... Because the concentrations of ATP, ADP, and Pi differ from one cell type to another, G for ATP hydrolysis likewise differs among cells. Moreover, in any given cell, G can vary from time to time, depending on the metabolic conditions in the cell and how they influence the concentrations of ATP, AD ...
... Because the concentrations of ATP, ADP, and Pi differ from one cell type to another, G for ATP hydrolysis likewise differs among cells. Moreover, in any given cell, G can vary from time to time, depending on the metabolic conditions in the cell and how they influence the concentrations of ATP, AD ...
2004 Lec 42-43: Nucleotide Metabolism
... No part of this presentation may be reproduced by any mechanical, photographic, or electronic process, or in the form of a phonographic recording, nor may it be stored in a retrieval system, transmitted, or otherwise copied for public or private use, without written permission from the publisher. ...
... No part of this presentation may be reproduced by any mechanical, photographic, or electronic process, or in the form of a phonographic recording, nor may it be stored in a retrieval system, transmitted, or otherwise copied for public or private use, without written permission from the publisher. ...
www.stat.tamu.edu
... Definition: Given the amino acid sequence of a protein, what is the protein's structure in three dimension? Importance: The structure of a protein provides a key to understanding its biological function. Assumption: The amino acid sequence contains all information about the native 3-D structure. The ...
... Definition: Given the amino acid sequence of a protein, what is the protein's structure in three dimension? Importance: The structure of a protein provides a key to understanding its biological function. Assumption: The amino acid sequence contains all information about the native 3-D structure. The ...
STRUCTURAL ORGANIZATION OF LIVING SYSTEMS At all levels
... Roles fulfilled by proteins in the cell are very diverse. To list just a few: • provide skeleton for the cell structure (structural proteins) • catalyze reactions (enzymes) • regulate processes (hormones) • actively transport molecules along elements of the cytoskeleton or across membranes • form ch ...
... Roles fulfilled by proteins in the cell are very diverse. To list just a few: • provide skeleton for the cell structure (structural proteins) • catalyze reactions (enzymes) • regulate processes (hormones) • actively transport molecules along elements of the cytoskeleton or across membranes • form ch ...
Lab 8 - Electrophoresis
... protein does not migrate in an electric field is called the isoelectric point. Most neutral amino acids have isoelectric points around pH 6.0. The isoelectric points of aspartic acid and glutamic acid, however, are close to pH 3. Therefore, at pH 6, these acidic amino acids carry a negative charge a ...
... protein does not migrate in an electric field is called the isoelectric point. Most neutral amino acids have isoelectric points around pH 6.0. The isoelectric points of aspartic acid and glutamic acid, however, are close to pH 3. Therefore, at pH 6, these acidic amino acids carry a negative charge a ...
22 CHEMISTRY OF ORGANIC COMPOUNDS Aims of the course
... Aims of the course: Students should become familiar with the basic principles of modern organic chemistry and understand their value in the function and reactivity of biomolecules as well as their interaction with small molecules. To this end, the chemistry and properties of the basic classes of org ...
... Aims of the course: Students should become familiar with the basic principles of modern organic chemistry and understand their value in the function and reactivity of biomolecules as well as their interaction with small molecules. To this end, the chemistry and properties of the basic classes of org ...
Lecture 4: Amino Acids
... Structural hierarchy in proteins • Primary structure (1º structure)-for a protein is the amino acid sequence of its polypeptide chain(s). • Secondary structure (2º structure)-the local spatial arrangement of a polypeptide’s backbone atoms without regard to the conformations of their side chains. • ...
... Structural hierarchy in proteins • Primary structure (1º structure)-for a protein is the amino acid sequence of its polypeptide chain(s). • Secondary structure (2º structure)-the local spatial arrangement of a polypeptide’s backbone atoms without regard to the conformations of their side chains. • ...
amino acids I-09 - ChemConnections
... Introduction to Biochemistry Most biologically important macromolecules are polymers, called biopolymers. Biopolymers fall into three classes: ...
... Introduction to Biochemistry Most biologically important macromolecules are polymers, called biopolymers. Biopolymers fall into three classes: ...
Answers - U of L Class Index
... The enzymes in glycolysis that are also used in their reverse directions for gluconeogenesis are phosphoglucoisomerase, aldolase, triosephosphate isomerase, glyceraldehyde 3-phosphate dehydrogenase, phosphoglycerokinase, phosphoglyceromutase, and enolase. ...
... The enzymes in glycolysis that are also used in their reverse directions for gluconeogenesis are phosphoglucoisomerase, aldolase, triosephosphate isomerase, glyceraldehyde 3-phosphate dehydrogenase, phosphoglycerokinase, phosphoglyceromutase, and enolase. ...
Browning - University of San Diego Home Pages
... The large brown molecules (caramelin, caramelen and caramelan) are what give caramel its color, its viscosity and its stickiness. The aroma molecules give caramel its flavor. The caramelization reactions require ...
... The large brown molecules (caramelin, caramelen and caramelan) are what give caramel its color, its viscosity and its stickiness. The aroma molecules give caramel its flavor. The caramelization reactions require ...
overview-omics - SRI International
... pathways, transporters, other membrane proteins, periplasmic proteins ...
... pathways, transporters, other membrane proteins, periplasmic proteins ...
Flower`n`Fruit
... Methionine: This is an ethylene precursor, which increases the quality and quantity of production. Proline: Its main function is to maintain the plant’s hydrous balance in the cell walls, resisting adverse conditions (drought, salinity, etc.). It increases the percentage of pollen grain germination, ...
... Methionine: This is an ethylene precursor, which increases the quality and quantity of production. Proline: Its main function is to maintain the plant’s hydrous balance in the cell walls, resisting adverse conditions (drought, salinity, etc.). It increases the percentage of pollen grain germination, ...
Photosynthesis
... • When RuBP is oxidized, it produces only 1 molecule of 3-PGA. • This process is called photorespiration. ...
... • When RuBP is oxidized, it produces only 1 molecule of 3-PGA. • This process is called photorespiration. ...
Biochemie jater
... 1. The liver takes up glucose and other monosaccharides from the blood plasma -These sugars are then converted to glucose 6-phosphate and other intermediates of glycolysis (subsequently, they are either stored as the reserve carbohydrate glycogen or degraded) -Another large part is converted into fa ...
... 1. The liver takes up glucose and other monosaccharides from the blood plasma -These sugars are then converted to glucose 6-phosphate and other intermediates of glycolysis (subsequently, they are either stored as the reserve carbohydrate glycogen or degraded) -Another large part is converted into fa ...
Metabolic Integration during the Postprandial, Fasting and Feedback
... and adipose tissue that accompany fasting [1,3,4,6,8]. It is needed to remember that the synthesis of glucose that occurs in the liver during periods of fasting the main precursors are amino acids, skeletal muscle, glycerol, resulting from the mobilization of adipose tissue triglycerides and Lactate ...
... and adipose tissue that accompany fasting [1,3,4,6,8]. It is needed to remember that the synthesis of glucose that occurs in the liver during periods of fasting the main precursors are amino acids, skeletal muscle, glycerol, resulting from the mobilization of adipose tissue triglycerides and Lactate ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.