Lipid metabolism
... which makes a difficulty in their digestion by the water-soluble lipases. Emulsification means breaking of large fat globules into small ones that increase the surface area to interaction with lipases and increase the rate of digestion. The emulsification is done mainly by bile salts. Lipases in ...
... which makes a difficulty in their digestion by the water-soluble lipases. Emulsification means breaking of large fat globules into small ones that increase the surface area to interaction with lipases and increase the rate of digestion. The emulsification is done mainly by bile salts. Lipases in ...
Balancing Redox Cofactor Generation and ATP Synthesis: Key
... of an ATP synthase subunit, alongside the observed shifts in the TCA cycle, suggested that an oxygen-scavenging electron transport chain likely remained active during low redox conditions. Together with the observed up-regulation of a glyoxalase and down-regulation of superoxide dismutase, thought t ...
... of an ATP synthase subunit, alongside the observed shifts in the TCA cycle, suggested that an oxygen-scavenging electron transport chain likely remained active during low redox conditions. Together with the observed up-regulation of a glyoxalase and down-regulation of superoxide dismutase, thought t ...
02-02_pptlect
... three fatty acids attached by dehydration synthesis to one molecule of glycerol ...
... three fatty acids attached by dehydration synthesis to one molecule of glycerol ...
Industrial biotechnology: Tools and applications
... Department of Chemical and Biomolecular Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, USA Departments of Chemistry, Biochemistry, and Bioengineering, Institute for Genomic Biology, University of Illinois at Urbana-Champaign, Urbana, IL, USA ...
... Department of Chemical and Biomolecular Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, USA Departments of Chemistry, Biochemistry, and Bioengineering, Institute for Genomic Biology, University of Illinois at Urbana-Champaign, Urbana, IL, USA ...
Gene expression: Translation
... The genetic code is a triplet code A set of 3 consecutive nucleotides make a codon in mRNA code, which corresponds to one amino acid in a polypeptide chain. ...
... The genetic code is a triplet code A set of 3 consecutive nucleotides make a codon in mRNA code, which corresponds to one amino acid in a polypeptide chain. ...
Metabolic Engineering VII: Health and Sustainability September 14-19, 2008 Program
... Sunday through Tuesday – El Patio Tent; Wednesday – on your own; Thursday – Vallarta Ballroom Poster Sessions/Social Hours – Vallarta Ballroom Thursday Reception: Vallarta Foyer Business Center: Computers for participant use ...
... Sunday through Tuesday – El Patio Tent; Wednesday – on your own; Thursday – Vallarta Ballroom Poster Sessions/Social Hours – Vallarta Ballroom Thursday Reception: Vallarta Foyer Business Center: Computers for participant use ...
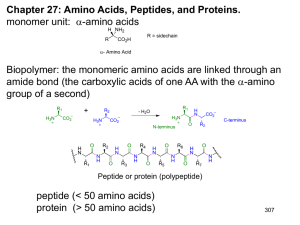
Chapter 3 Amino Acids, Peptides, Proteins
... condensed into peptide (small) protein (large) Both can be degraded back to AA’s by hydrolysis (At neutral pH hydrolysis is slow but faster in acid or base Large bulk of proteins broken down to 20 different amino acids A. Amino acids share Common structural features. i. C atom center, Amino group on ...
... condensed into peptide (small) protein (large) Both can be degraded back to AA’s by hydrolysis (At neutral pH hydrolysis is slow but faster in acid or base Large bulk of proteins broken down to 20 different amino acids A. Amino acids share Common structural features. i. C atom center, Amino group on ...
ppt - Vanderbilt University
... Enzymes: proteins that catalyze biochemical reactions. • by bringing the reactive atoms together in the optimal geometry for the reaction. • lowering the activation energy (G‡) by stabilizing the transition state and/or high energy intermediate. • many enzymes use the functional groups of the amin ...
... Enzymes: proteins that catalyze biochemical reactions. • by bringing the reactive atoms together in the optimal geometry for the reaction. • lowering the activation energy (G‡) by stabilizing the transition state and/or high energy intermediate. • many enzymes use the functional groups of the amin ...
Carbonyl Chemistry - Fundamentals
... o carbonyl carbon is bonded to two alkyl (or aryl) groups - replace “e” from the name of the parent hydrocarbon and adding “one” - the chain is numbered in the direction that gives the carbonyl carbon the ...
... o carbonyl carbon is bonded to two alkyl (or aryl) groups - replace “e” from the name of the parent hydrocarbon and adding “one” - the chain is numbered in the direction that gives the carbonyl carbon the ...
13lctout - Evergreen Archives
... A. The protein-coding region of eukaryotic genes is interrupted by stretches of noncoding DNA. 1. Noncoding sequences must be disposed of to make a functional mRNA. 2. Eukaryotic gene organization is very different from that in prokaryotes. B. P. Sharp et al. detected noncoding regions in genes of t ...
... A. The protein-coding region of eukaryotic genes is interrupted by stretches of noncoding DNA. 1. Noncoding sequences must be disposed of to make a functional mRNA. 2. Eukaryotic gene organization is very different from that in prokaryotes. B. P. Sharp et al. detected noncoding regions in genes of t ...
Slide 1
... from Samson, W.K. and Resch, Z.T., Trends in Endocrinology & Metabolism, 11, 257–262, 2000. © Elsevier Science, Inc.) Copyright © 2013 Elsevier Inc. All rights reserved. ...
... from Samson, W.K. and Resch, Z.T., Trends in Endocrinology & Metabolism, 11, 257–262, 2000. © Elsevier Science, Inc.) Copyright © 2013 Elsevier Inc. All rights reserved. ...
Supplementary Information (doc 662K)
... EDTA, 2 mM dithiothreitol (DTT), 5% glycerol. The protein was then applied to a SP HP column at rate of 2 ml/min. The protein was then eluted with a linear gradient of 0.15-0.8 M NaCl in buffer containing 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 2 mM DTT, and 5% glycerol. The fractions containing the p ...
... EDTA, 2 mM dithiothreitol (DTT), 5% glycerol. The protein was then applied to a SP HP column at rate of 2 ml/min. The protein was then eluted with a linear gradient of 0.15-0.8 M NaCl in buffer containing 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 2 mM DTT, and 5% glycerol. The fractions containing the p ...
Chapter 21: Molecules of Life - Follow “Ironmtn.wordpress.com”
... the polyunsaturated foods to improve texture and consistency. Hydrogenation eliminates some of the double bonds by adding hydrogen atoms back onto the carbon chain and changing the lipids from polyunsaturated to partially saturated. Link To: Lipids Difficulty Level: Hard ...
... the polyunsaturated foods to improve texture and consistency. Hydrogenation eliminates some of the double bonds by adding hydrogen atoms back onto the carbon chain and changing the lipids from polyunsaturated to partially saturated. Link To: Lipids Difficulty Level: Hard ...
Microbiology
... Are dominated by methanogens - All are poisoned by molecular oxygen and therefore require complete anaerobiosis. - Major substrates and reactions include: Carbon dioxide: CO2 + 4H2 → CH4 + 2H2O Acetic acid: CH3COOH → CH4 + CO2 Methanol: 4CH3OH → 3CH4 + CO2 + 2H2O ...
... Are dominated by methanogens - All are poisoned by molecular oxygen and therefore require complete anaerobiosis. - Major substrates and reactions include: Carbon dioxide: CO2 + 4H2 → CH4 + 2H2O Acetic acid: CH3COOH → CH4 + CO2 Methanol: 4CH3OH → 3CH4 + CO2 + 2H2O ...
lecture7
... Fatty acid synthesis starts with the carboxylation of acetyl CoA to malonyl CoA. This irreversible reaction is the committed step in fatty acid synthesis. The synthesis of malonyl CoA is catalyzed by acetyl CoA carboxylase, which contains a biotin prosthetic group. The carboxyl group of biotin is co ...
... Fatty acid synthesis starts with the carboxylation of acetyl CoA to malonyl CoA. This irreversible reaction is the committed step in fatty acid synthesis. The synthesis of malonyl CoA is catalyzed by acetyl CoA carboxylase, which contains a biotin prosthetic group. The carboxyl group of biotin is co ...
Fatty acid oxidation and the P-oxidation complex in
... pathogenic for mice. These strains were grown in experimental animals as well as axenically with and ...
... pathogenic for mice. These strains were grown in experimental animals as well as axenically with and ...
ppt
... Ch. 23 Oxidation of fatty acids, ketones Student Learning Outcomes: • Explain how fatty acids are a major fuel source, especially after fasting • Explain how liver makes ketone bodies, fuel for other cells • Describe the basic categories of fatty acids: VLC, LC, med • Explain b-oxidation pathway of ...
... Ch. 23 Oxidation of fatty acids, ketones Student Learning Outcomes: • Explain how fatty acids are a major fuel source, especially after fasting • Explain how liver makes ketone bodies, fuel for other cells • Describe the basic categories of fatty acids: VLC, LC, med • Explain b-oxidation pathway of ...
biogenic s, p, d-block elements, biological role, application in medicine
... Hydrogen is used in such industries as: chemical industry (production of ammonia, methanol, soap and plastic), food industry (registered as a food additive E949), and aviation industry. Electron configuration of atoms of hydrogen is 1s1. Hydrogen similar to alkaline metals is univalent and has redu ...
... Hydrogen is used in such industries as: chemical industry (production of ammonia, methanol, soap and plastic), food industry (registered as a food additive E949), and aviation industry. Electron configuration of atoms of hydrogen is 1s1. Hydrogen similar to alkaline metals is univalent and has redu ...
3. d-Block elements. Biological role, application in medicine.
... Hydrogen is used in such industries as: chemical industry (production of ammonia, methanol, soap and plastic), food industry (registered as a food additive E949), and aviation industry. Electron configuration of atoms of hydrogen is 1s1. Hydrogen similar to alkaline metals is univalent and has redu ...
... Hydrogen is used in such industries as: chemical industry (production of ammonia, methanol, soap and plastic), food industry (registered as a food additive E949), and aviation industry. Electron configuration of atoms of hydrogen is 1s1. Hydrogen similar to alkaline metals is univalent and has redu ...
(CH14) Translation (Slides)
... Viral double-stranded RNA binds to the RNA binding domains of PKR. PKR catalytic-domain dimerization. Autophosphorylation of PKR. Specific recognition of eIF2. Phosphorylation of eIF2. ...
... Viral double-stranded RNA binds to the RNA binding domains of PKR. PKR catalytic-domain dimerization. Autophosphorylation of PKR. Specific recognition of eIF2. Phosphorylation of eIF2. ...
Exam II ReviewQuestions
... 13. Liver alcohol dehydrogenases (ADH) is relatively nonspecific. Its normal substrate is ethanol, however, it will oxidize other primary alcohols, such as methanol, to their corresponding aldehydes. In the case of methanol this produces formaldehyde, which is quite toxic and can lead to blindness. ...
... 13. Liver alcohol dehydrogenases (ADH) is relatively nonspecific. Its normal substrate is ethanol, however, it will oxidize other primary alcohols, such as methanol, to their corresponding aldehydes. In the case of methanol this produces formaldehyde, which is quite toxic and can lead to blindness. ...
BIO121_Chapter 6
... Cellular Respiration of One Glucose Yields 36 ATP Glycolysis and Krebs cycle each produce 2 ATP, and the electron transport chain produces 34 ATP. Transporting NADH into the mitochondrion requires 2 ATP, making the total production of ATP equal to 36. ...
... Cellular Respiration of One Glucose Yields 36 ATP Glycolysis and Krebs cycle each produce 2 ATP, and the electron transport chain produces 34 ATP. Transporting NADH into the mitochondrion requires 2 ATP, making the total production of ATP equal to 36. ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.