File - Dr. Venables` Chemistry Sites
... 1. Write down the two half reactions. 2. Balance each half reaction: a. First with elements other than H and O. b. Then balance O by adding water. c. Then balance H by adding H+. d. If it is in basic solution, remove H+ by adding OHe. Finish by balancing charge by adding electrons. ...
... 1. Write down the two half reactions. 2. Balance each half reaction: a. First with elements other than H and O. b. Then balance O by adding water. c. Then balance H by adding H+. d. If it is in basic solution, remove H+ by adding OHe. Finish by balancing charge by adding electrons. ...
Monte Carlo Methods in Scientific Computing
... X=(x1,x2, …, xi, …, xN), and if we can compute P(xi|x1,…,xi-1,xi+1,..,xN), we generate the new configuration by sampling xi according to the above ...
... X=(x1,x2, …, xi, …, xN), and if we can compute P(xi|x1,…,xi-1,xi+1,..,xN), we generate the new configuration by sampling xi according to the above ...
Balancing Chemical Equation Practice.docx
... Reading adapted from Sarquis’s Modern Chemistry Introduction A chemical reaction is the process by which one or more substances are changed into one or more different substances. In any chemical reaction, the original substances are known as the reactants, and the resulting substances are known as t ...
... Reading adapted from Sarquis’s Modern Chemistry Introduction A chemical reaction is the process by which one or more substances are changed into one or more different substances. In any chemical reaction, the original substances are known as the reactants, and the resulting substances are known as t ...
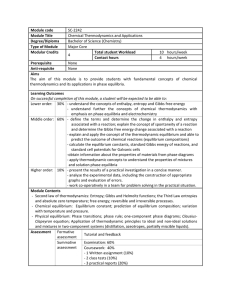
Module code SC-2242 Module Title Chemical Thermodynamics and
... Lower order: 30% - understand the concepts of enthalpy, entropy and Gibbs free energy - understand further the concepts of chemical thermodynamics with emphasis on phase equilibria and electrochemistry Middle order: 60% - define the terms and determine the change in enthalpy ...
... Lower order: 30% - understand the concepts of enthalpy, entropy and Gibbs free energy - understand further the concepts of chemical thermodynamics with emphasis on phase equilibria and electrochemistry Middle order: 60% - define the terms and determine the change in enthalpy ...
ChE 215, Physical Chemistry
... The objective of this course is to introduce the basic topics in Kinetics, Thermodynamics and Statistical Mechanics. In fact this course present collection of distinct topics particularly useful to chemical engineering students. The basic principles in the course are demonstrated by six experiments ...
... The objective of this course is to introduce the basic topics in Kinetics, Thermodynamics and Statistical Mechanics. In fact this course present collection of distinct topics particularly useful to chemical engineering students. The basic principles in the course are demonstrated by six experiments ...
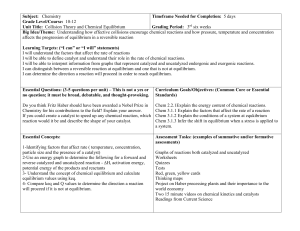
Equilibrium and Kinetics
... 10. Derive an expression for the variation of reactant concentration with respect to time for a reaction which exhibits zero order kinetics. How can this expression be used graphically to evaluate the rate constant? 11. Sketch and label the phase diagram of Sulphur system. 12. Differentiate between ...
... 10. Derive an expression for the variation of reactant concentration with respect to time for a reaction which exhibits zero order kinetics. How can this expression be used graphically to evaluate the rate constant? 11. Sketch and label the phase diagram of Sulphur system. 12. Differentiate between ...
Syllabus
... Grading Policy: There will be 12 homework assignments, a midterm and a final. All are given on a take-home basis. If you have any question about a problem before starting to work, you are strongly encouraged to discuss the matter with the professor or fellow students. That is, students are encourage ...
... Grading Policy: There will be 12 homework assignments, a midterm and a final. All are given on a take-home basis. If you have any question about a problem before starting to work, you are strongly encouraged to discuss the matter with the professor or fellow students. That is, students are encourage ...
Chemical Equations
... Simple combustion reactions involve the reaction of hydrocarbons with oxygen to form carbon dioxide and water. Balance the C, H and O atoms in that order. If, in the end, there is an odd number of O atoms on the right, you may need to double the hydrocarbon by simply placing a 2 coefficient in front ...
... Simple combustion reactions involve the reaction of hydrocarbons with oxygen to form carbon dioxide and water. Balance the C, H and O atoms in that order. If, in the end, there is an odd number of O atoms on the right, you may need to double the hydrocarbon by simply placing a 2 coefficient in front ...