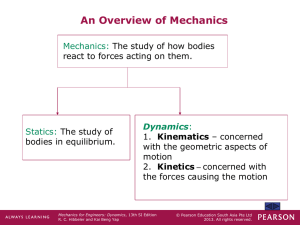
week 1
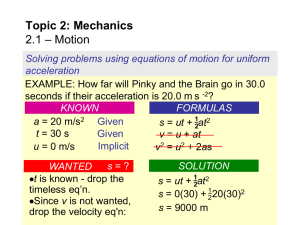
... The position of the particle at any instant, relative to the origin, O, is defined by the position vector r, or the scalar s. Scalar s can be positive or negative. Typical units for r and s are meters (m) or feet (ft). The displacement of the particle is defined as its change in position. ...
... The position of the particle at any instant, relative to the origin, O, is defined by the position vector r, or the scalar s. Scalar s can be positive or negative. Typical units for r and s are meters (m) or feet (ft). The displacement of the particle is defined as its change in position. ...
Entropy Notes II
... Third Law of Thermodynamics • The entropy of a perfect/pure crystalline solid is zero at a temp of 0 K (absolute zero) – Perfect order ...
... Third Law of Thermodynamics • The entropy of a perfect/pure crystalline solid is zero at a temp of 0 K (absolute zero) – Perfect order ...
Contents - MyCourses
... of matter – largely independent of models of microscopic structure (which where practically nonexistent at the time of foundation of thermodynamics in the 19th century). It is based on very few basic laws plus rules of calculus. Properties of matter or concrete systems are taken from outside (experi ...
... of matter – largely independent of models of microscopic structure (which where practically nonexistent at the time of foundation of thermodynamics in the 19th century). It is based on very few basic laws plus rules of calculus. Properties of matter or concrete systems are taken from outside (experi ...
AH (SHM) - mrmackenzie
... 10) The displacement-time graphs for four different objects undergoing simple harmonic motion are shown. In each case: (a) Determine the value for the amplitude (A), period (T), frequency (f) and angular frequency (ω ω) of the motion. (b) Use values from part (a) to obtain an expression in the form ...
... 10) The displacement-time graphs for four different objects undergoing simple harmonic motion are shown. In each case: (a) Determine the value for the amplitude (A), period (T), frequency (f) and angular frequency (ω ω) of the motion. (b) Use values from part (a) to obtain an expression in the form ...
ENGR-36_Lec-02_Fa12_Forces_as_Vectors_
... This Force Exerted by the Earth is called Weight • While g Varies Somewhat With the Elevation & Location, to a Very Good Approximation – g 9.81 m/s2 32.2 ft/s2 Engineering-36: Engineering Mechanics - Statics ...
... This Force Exerted by the Earth is called Weight • While g Varies Somewhat With the Elevation & Location, to a Very Good Approximation – g 9.81 m/s2 32.2 ft/s2 Engineering-36: Engineering Mechanics - Statics ...
Document
... 1) The object returns to its original position, in which case it is said to be in Stable equilibrium; 2) The object moves even father from its original position, in which case it is said to be in unstable equilibrium; 3) And, The object remains in its new position, in which case it is said to be in ...
... 1) The object returns to its original position, in which case it is said to be in Stable equilibrium; 2) The object moves even father from its original position, in which case it is said to be in unstable equilibrium; 3) And, The object remains in its new position, in which case it is said to be in ...
Lecture 8 - Carrier Drift and Diffusion (cont
... • What would that imply for the electron and hole currents? • Is there a relationship between mobility and diffusion coefficient? • Given a certain non-uniform doping distribution, how does one compute the equilibrium carrier concentrations? • Under what conditions does the equilibrium majority carr ...
... • What would that imply for the electron and hole currents? • Is there a relationship between mobility and diffusion coefficient? • Given a certain non-uniform doping distribution, how does one compute the equilibrium carrier concentrations? • Under what conditions does the equilibrium majority carr ...
I. Development of the Virial Theorem
... Lagrange's identity in a variety of ways, but have not rigorously taken that finial step to produce the virial theorem. This last step involves averaging over time and it is in this form that the theorem finds its widest application. However, in astrophysics few if any investigators live long enough ...
... Lagrange's identity in a variety of ways, but have not rigorously taken that finial step to produce the virial theorem. This last step involves averaging over time and it is in this form that the theorem finds its widest application. However, in astrophysics few if any investigators live long enough ...
2007 The McGraw-Hill Companies, Inc. All rights reserved. 13
... • Force acting on a particle during a very short time interval that is large enough to cause a significant change in momentum is called an impulsive force. • When impulsive forces act on a particle, ...
... • Force acting on a particle during a very short time interval that is large enough to cause a significant change in momentum is called an impulsive force. • When impulsive forces act on a particle, ...
March 26, 2013 Palmetto Lecture on Comparative Inference
... Theorem 1 suggests that the Bayes estimator will be superior to θb unless the Bayesian statistician miscalculates on two fronts simultaneously. If a Bayesian is both misguided (with a poorly centered prior) and stubborn (with a prior that is highly concentrated on the prior guess), his estimation pe ...
... Theorem 1 suggests that the Bayes estimator will be superior to θb unless the Bayesian statistician miscalculates on two fronts simultaneously. If a Bayesian is both misguided (with a poorly centered prior) and stubborn (with a prior that is highly concentrated on the prior guess), his estimation pe ...
THE KINETICS OF CHEMICAL REACTIONS: SINGLE
... them are shown). Each molecule jumps randomly between the states A and B. I have generated a trajectory for each on the computer using a simple random number generator (a computer program that spits out random numbers). Of course I had to know some additional information: For example, it is necessar ...
... them are shown). Each molecule jumps randomly between the states A and B. I have generated a trajectory for each on the computer using a simple random number generator (a computer program that spits out random numbers). Of course I had to know some additional information: For example, it is necessar ...