Slide1
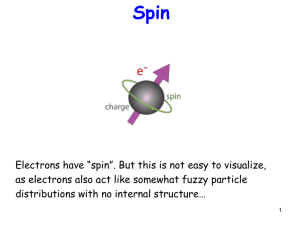
... Electrons have “spin”. But this is not easy to visualize, as electrons also act like somewhat fuzzy particle distributions with no internal structure… ...
... Electrons have “spin”. But this is not easy to visualize, as electrons also act like somewhat fuzzy particle distributions with no internal structure… ...
A tutorial on non-Markovian quantum processes
... we characterise non-Markovian quantum processes? If so, how? ...
... we characterise non-Markovian quantum processes? If so, how? ...
Lecture 9: Macroscopic Quantum Model
... The physical meaning of the wave function Ψ The absolute phase of a plane wave should not influence the overall physics of a system. So Max Born hypothesized in ~1927 that the square of the magnitude of the wave function Ψ was equal to the probability of a quantum mechanical particle to be at the l ...
... The physical meaning of the wave function Ψ The absolute phase of a plane wave should not influence the overall physics of a system. So Max Born hypothesized in ~1927 that the square of the magnitude of the wave function Ψ was equal to the probability of a quantum mechanical particle to be at the l ...
Quantum Computing Devices Quantum Bits
... If M1 and M2 are 2 x 2 matrices that describe unitary quantum gates, then it is easy to verify that the joint actions of M1 of the first qubis and M2 on the second are described by M1 ⊗ M2 This generalize to quantum systems of any size If matrices M1 and M2 define unitary mappings on Hilbert soace ...
... If M1 and M2 are 2 x 2 matrices that describe unitary quantum gates, then it is easy to verify that the joint actions of M1 of the first qubis and M2 on the second are described by M1 ⊗ M2 This generalize to quantum systems of any size If matrices M1 and M2 define unitary mappings on Hilbert soace ...
Lecture 4 — January 14, 2016 1 Outline 2 Weyl
... Let us consider the description of a simple physical system, such as an electron. From a classical point of view, the physical state of the electron at any instant is characterized by six quantities, that is, its three position coordinates, and its three momentum coordinates. In other words, to each ...
... Let us consider the description of a simple physical system, such as an electron. From a classical point of view, the physical state of the electron at any instant is characterized by six quantities, that is, its three position coordinates, and its three momentum coordinates. In other words, to each ...
The quantum does not reduce to discrete bits
... described above. Sometimes the textbooks admit that the quantum probabilities require special interpretation because they can be negative. I say that negative probabilities are not probabilities and that the probabilities are no more essential to quantum mechanics than to any other physical theory t ...
... described above. Sometimes the textbooks admit that the quantum probabilities require special interpretation because they can be negative. I say that negative probabilities are not probabilities and that the probabilities are no more essential to quantum mechanics than to any other physical theory t ...
Statistics, Causality and Bell`s theorem
... Fortunately, one can understand quite a lot of (5) without any understanding of quantum mechanics: we just need to know certain simple statistical predictions which follow from a particular special model in quantum physics called the EPR-B model. The initials refer here to the celebrated paradox of ...
... Fortunately, one can understand quite a lot of (5) without any understanding of quantum mechanics: we just need to know certain simple statistical predictions which follow from a particular special model in quantum physics called the EPR-B model. The initials refer here to the celebrated paradox of ...
powerpoint
... How Can We Distinguish Between the Two? By measuring another property: the probability of a reaction in different angles. The angular dependency of the steric factor in a nucleofilic charge reaction is examined. The basis set of the direction measurements differentiates between superposition and a ...
... How Can We Distinguish Between the Two? By measuring another property: the probability of a reaction in different angles. The angular dependency of the steric factor in a nucleofilic charge reaction is examined. The basis set of the direction measurements differentiates between superposition and a ...
quantum mechanical model
... to higher energy levels. This is called the excited state. • When the electron returns to the ground state, it releases energy in the form of light. Emission Line Spectra ...
... to higher energy levels. This is called the excited state. • When the electron returns to the ground state, it releases energy in the form of light. Emission Line Spectra ...