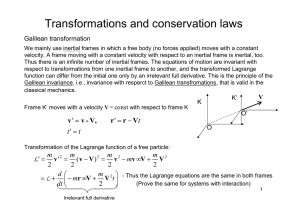
Homework 3 - barnes report
... under “Normal distribution” or “Error function” for the numerical value of the integral. ...
... under “Normal distribution” or “Error function” for the numerical value of the integral. ...
Matthew Jones - Phys 378 Web page:
... Are there only four forces? Is the model that describes them self-consistent? Why does nature look this way? Are there cosmological implications? We think we might get some answers in the next decade ...
... Are there only four forces? Is the model that describes them self-consistent? Why does nature look this way? Are there cosmological implications? We think we might get some answers in the next decade ...
Introduction
... – Interval scale: ranked, and space between scores are the same size – Ratio, ranked, space between scores are the same size, have absolute zero point ...
... – Interval scale: ranked, and space between scores are the same size – Ratio, ranked, space between scores are the same size, have absolute zero point ...
PY 482: Computation for Experimental Particle Physics
... • Familiarity with C++ and UNIX 4. Course Objectives: To provide an introduction to the experimental methods used in modern high energy particle physics. The course will start with a survey of modern experimental particle physics where students will familiarize themselves with the Standard Model of ...
... • Familiarity with C++ and UNIX 4. Course Objectives: To provide an introduction to the experimental methods used in modern high energy particle physics. The course will start with a survey of modern experimental particle physics where students will familiarize themselves with the Standard Model of ...
Quantum and Atomic Physics
... (B) The interference pattern produced by neutrons incident on a crystal (C) The production of x-rays by electrons striking a metal target (D) Compton scattering 23. Which of the following formulas can be used to determine the de Broglie wavelength? (A) λ = hmv (B) λ = h/mv (C) λ = mv/h (D) λ = mc/h ...
... (B) The interference pattern produced by neutrons incident on a crystal (C) The production of x-rays by electrons striking a metal target (D) Compton scattering 23. Which of the following formulas can be used to determine the de Broglie wavelength? (A) λ = hmv (B) λ = h/mv (C) λ = mv/h (D) λ = mc/h ...
The Second Law of Thermodynamics
... The physical and chemical properties of elements is determined by the atomic structure. The atomic structure is, in turn, determined by the electrons and which shells, subshells and orbitals they reside in. The rules of placing electrons within shells is known as the Aufbau principle. As protons are ...
... The physical and chemical properties of elements is determined by the atomic structure. The atomic structure is, in turn, determined by the electrons and which shells, subshells and orbitals they reside in. The rules of placing electrons within shells is known as the Aufbau principle. As protons are ...
Modern physics
... Cyclotron frequency: An electron moving with speed v perpendicular to an external magnetic field feels a Lorentz ...
... Cyclotron frequency: An electron moving with speed v perpendicular to an external magnetic field feels a Lorentz ...
A1980KM40500001
... transformed so that, for a completely unsymmetrical molecule, there are only (n+ 1) terms for each even degree n. [The SCI ® indicates that this paper has been cited over 305 times since 1967.] James K. G. Watson Department of Chemistry University of Southampton Southampton SO9 5NH England September ...
... transformed so that, for a completely unsymmetrical molecule, there are only (n+ 1) terms for each even degree n. [The SCI ® indicates that this paper has been cited over 305 times since 1967.] James K. G. Watson Department of Chemistry University of Southampton Southampton SO9 5NH England September ...
Presentation (PowerPoint File)
... decoherence through error correction and fault-tolerant computation. 5. Measurement: The ability to read out the state of the computer in a convenient product basis. ...
... decoherence through error correction and fault-tolerant computation. 5. Measurement: The ability to read out the state of the computer in a convenient product basis. ...
Quantum Mechanics
... We know some small particles like electrons, protons, and neutrons. Quantum physics even describes the particles which make these particles! (The model of an atom that you were taught in high-school is a approximation). The electrons don't orbit like planets; they form blurred clouds of probabiliti ...
... We know some small particles like electrons, protons, and neutrons. Quantum physics even describes the particles which make these particles! (The model of an atom that you were taught in high-school is a approximation). The electrons don't orbit like planets; they form blurred clouds of probabiliti ...
6. Quantum Mechanics II
... Note that more than one wave function can have the same energy. When more than one wave function has the same energy, those quantum states are said to be degenerate. Degeneracy results from symmetries of the potential energy function that describes the system. A perturbation of the potential energy ...
... Note that more than one wave function can have the same energy. When more than one wave function has the same energy, those quantum states are said to be degenerate. Degeneracy results from symmetries of the potential energy function that describes the system. A perturbation of the potential energy ...
On the Quantum Aspects of Geophysics
... According to quantum theory, every mass m, with momentum p has a wavelength λ = hp , where h is the planck constant. In quantum cosmology the universe is simply assumed to be a particle in a definite potential and one usually takes the units so that h = 1. This is because people are usually interest ...
... According to quantum theory, every mass m, with momentum p has a wavelength λ = hp , where h is the planck constant. In quantum cosmology the universe is simply assumed to be a particle in a definite potential and one usually takes the units so that h = 1. This is because people are usually interest ...
eprint_11_28683_250
... position of the electron at the same instant in time. This is a statement of Heisenberg’s uncertainty principle. In order to get around this problem, rather than trying to define its exact position and momentum, we use the probability of finding the electron in a given volume of space. The probabil ...
... position of the electron at the same instant in time. This is a statement of Heisenberg’s uncertainty principle. In order to get around this problem, rather than trying to define its exact position and momentum, we use the probability of finding the electron in a given volume of space. The probabil ...
PowerPoint
... Bohr’s theory correctly explained the hydrogen emission spectrum The theory failed for all other elements with more than 1 electron Bohr’s theory attempted to use classical mechanics to solve a problem that could not be solved by classical mechanics ...
... Bohr’s theory correctly explained the hydrogen emission spectrum The theory failed for all other elements with more than 1 electron Bohr’s theory attempted to use classical mechanics to solve a problem that could not be solved by classical mechanics ...
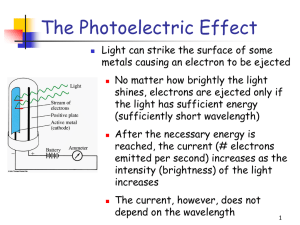
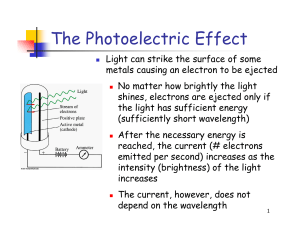
The Photoelectric Effect
... Bohr’s theory correctly explained the hydrogen emission spectrum The theory failed for all other elements with more than 1 electron Bohr’s theory attempted to use classical mechanics to solve a problem that could not be solved by classical mechanics ...
... Bohr’s theory correctly explained the hydrogen emission spectrum The theory failed for all other elements with more than 1 electron Bohr’s theory attempted to use classical mechanics to solve a problem that could not be solved by classical mechanics ...
Renormalization group

In theoretical physics, the renormalization group (RG) refers to a mathematical apparatus that allows systematic investigation of the changes of a physical system as viewed at different distance scales. In particle physics, it reflects the changes in the underlying force laws (codified in a quantum field theory) as the energy scale at which physical processes occur varies, energy/momentum and resolution distance scales being effectively conjugate under the uncertainty principle (cf. Compton wavelength).A change in scale is called a ""scale transformation"". The renormalization group is intimately related to ""scale invariance"" and ""conformal invariance"", symmetries in which a system appears the same at all scales (so-called self-similarity). (However, note that scale transformations are included in conformal transformations, in general: the latter including additional symmetry generators associated with special conformal transformations.)As the scale varies, it is as if one is changing the magnifying power of a notional microscope viewing the system. In so-called renormalizable theories, the system at one scale will generally be seen to consist of self-similar copies of itself when viewed at a smaller scale, with different parameters describing the components of the system. The components, or fundamental variables, may relate to atoms, elementary particles, atomic spins, etc. The parameters of the theory typically describe the interactions of the components. These may be variable ""couplings"" which measure the strength of various forces, or mass parameters themselves. The components themselves may appear to be composed of more of the self-same components as one goes to shorter distances.For example, in quantum electrodynamics (QED), an electron appears to be composed of electrons, positrons (anti-electrons) and photons, as one views it at higher resolution, at very short distances. The electron at such short distances has a slightly different electric charge than does the ""dressed electron"" seen at large distances, and this change, or ""running,"" in the value of the electric charge is determined by the renormalization group equation.