64-311/5: Atomic and Molecular Spectra
... The Schrodinger model for hydrogen agreed with the Bohr model in that they both resulted in the energies of the excited states being only a function of principal quantum number n. This 'accidental degeneracy' for the different l states is not strictly true as closer scrutiny of the spectral lines re ...
... The Schrodinger model for hydrogen agreed with the Bohr model in that they both resulted in the energies of the excited states being only a function of principal quantum number n. This 'accidental degeneracy' for the different l states is not strictly true as closer scrutiny of the spectral lines re ...
AOW- Time Travel
... that time travel is possible would have change classical physics as well as allow for super-fast types of computing that rely on quantum physics, also called quantum mechanics. Briefly, classical physics deals with the big things, like the Sun and Moon. Quantum mechanics tells us that the things des ...
... that time travel is possible would have change classical physics as well as allow for super-fast types of computing that rely on quantum physics, also called quantum mechanics. Briefly, classical physics deals with the big things, like the Sun and Moon. Quantum mechanics tells us that the things des ...
Physics 235 Chapter 8 Central-Force Motion
... In general, this equation has two solutions, and the orbit is confined between a minimum and maximum value of r. Under certain conditions, there is only a single solution, and in that case the orbit is circular. Using the orbital equation we can determine the change in the polar angle when the radiu ...
... In general, this equation has two solutions, and the orbit is confined between a minimum and maximum value of r. Under certain conditions, there is only a single solution, and in that case the orbit is circular. Using the orbital equation we can determine the change in the polar angle when the radiu ...
Sample pages 1 PDF
... movement of a neutron with the same speed (103 m/s) is associated with a wave of wavelength λ ≈ 4 × 10−13 m. In other words, a neutron moving with that speed can be considered a de Broglie wave with a wavelength of 4 × 10−13 m. Similar wavelengths are characteristic of cosmic rays. Particles with a ...
... movement of a neutron with the same speed (103 m/s) is associated with a wave of wavelength λ ≈ 4 × 10−13 m. In other words, a neutron moving with that speed can be considered a de Broglie wave with a wavelength of 4 × 10−13 m. Similar wavelengths are characteristic of cosmic rays. Particles with a ...
Department of Physics and Astronomy University of Georgia
... (a) Solve the time-independent 1D Schrödinger equation for this potential, and find the forward and backward scattering amplitudes in terms of Ω. (Hint: consider what are the appropriate boundary conditions for the wavefunction at x=0.) (b) Show that for Ω → ∞ (strong potential), the particle is tot ...
... (a) Solve the time-independent 1D Schrödinger equation for this potential, and find the forward and backward scattering amplitudes in terms of Ω. (Hint: consider what are the appropriate boundary conditions for the wavefunction at x=0.) (b) Show that for Ω → ∞ (strong potential), the particle is tot ...
1 - Penn Math
... A cyclist is on a long straight road leading from her home to a nearby lake. Assume that the speed of travel toward the lake is taken as positive (and speed toward home is negative) The graph below shows the cyclist’s speed as a function of time. She reaches the lake at the point labeled A, stops fo ...
... A cyclist is on a long straight road leading from her home to a nearby lake. Assume that the speed of travel toward the lake is taken as positive (and speed toward home is negative) The graph below shows the cyclist’s speed as a function of time. She reaches the lake at the point labeled A, stops fo ...
physical chemistry ii chem 3354
... • Quantum mechanics was discovered in the 1920s to describe very small particles. – The basis of quantum mechanics is that energy is quantized – has discreet values. – Particles have wave-like characteristics. ...
... • Quantum mechanics was discovered in the 1920s to describe very small particles. – The basis of quantum mechanics is that energy is quantized – has discreet values. – Particles have wave-like characteristics. ...
Mod 2 - Manhasset Public Schools
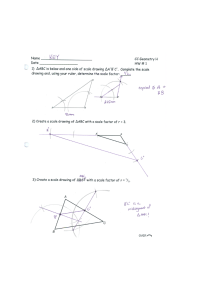
... 1. Use your ruler to draw a ray from O, passing through each vertex of the figure. 2. Measure the distance between O and a vertex, and multiply it by the scale factor. The resulting value is the distance away from O at which the scaled point will be located. 3. Once all the vertices are dilated, joi ...
... 1. Use your ruler to draw a ray from O, passing through each vertex of the figure. 2. Measure the distance between O and a vertex, and multiply it by the scale factor. The resulting value is the distance away from O at which the scaled point will be located. 3. Once all the vertices are dilated, joi ...
Atomic Theory Notes
... Because we cannot see atoms, we use models to teach and learn about atoms. The atomic theory has changed over time as new technologies have become available. o Remember: Scientific knowledge builds on past research and experimentation. ...
... Because we cannot see atoms, we use models to teach and learn about atoms. The atomic theory has changed over time as new technologies have become available. o Remember: Scientific knowledge builds on past research and experimentation. ...
Matrix model formulations of superstring theory
... In naïve quantum extension of Einstein’s theory : UV divergence becomes worse at higher orders in the expansion w.r.t. the coupling constant ! ...
... In naïve quantum extension of Einstein’s theory : UV divergence becomes worse at higher orders in the expansion w.r.t. the coupling constant ! ...
Chapter 7
... electrons varies linearly with the frequency of the incident radiation but is independent of its intensity. • Even at low intensities of light, electrons are ejected immediately if the frequency is above the threshold value. ...
... electrons varies linearly with the frequency of the incident radiation but is independent of its intensity. • Even at low intensities of light, electrons are ejected immediately if the frequency is above the threshold value. ...
Jokipii-CRacc
... BUT, what happens at high energies? • We must remember that observations mandate that L scale as T.6. • This gives ¼ 1 at 1018 eV • Observations give < ¼ 5% (Sokolsky, private communication, 2007. • The theoretical scaling of L as T.33 for Kolmogorov turbulence is barely acceptable at about 5 ...
... BUT, what happens at high energies? • We must remember that observations mandate that L scale as T.6. • This gives ¼ 1 at 1018 eV • Observations give < ¼ 5% (Sokolsky, private communication, 2007. • The theoretical scaling of L as T.33 for Kolmogorov turbulence is barely acceptable at about 5 ...
Geometry Notes G.7 Similar Polygons and Triangles Mrs. Grieser
... Name: _______________________________ Date: _____________ Block: _______ Congruence vs. Similarity ...
... Name: _______________________________ Date: _____________ Block: _______ Congruence vs. Similarity ...
PHYS 2100 Final Examination Introduction to Methods of Theoretical Physics Fall 1998
... There is an infinitely high potential wall at x = 0 and the potential remains at zero for x → ∞ . The particle is in a bound, stationary quantum mechanical state. a) Sketch a possible energy level on the diagram above. b) Sketch a possible form for the time-independent wave function u ( x ) using th ...
... There is an infinitely high potential wall at x = 0 and the potential remains at zero for x → ∞ . The particle is in a bound, stationary quantum mechanical state. a) Sketch a possible energy level on the diagram above. b) Sketch a possible form for the time-independent wave function u ( x ) using th ...
The Emergence of a Macro-World: A Study of Intertheory Relations in Classical and Quantum Mechanics
... definition, means that the total kinetic energy is conserved), then the final state of the colliding particle after the interaction is fully determined from its initial state by the conservation of total momentum and total energy. This is true irrespective of the exact nature of the forces involved in ...
... definition, means that the total kinetic energy is conserved), then the final state of the colliding particle after the interaction is fully determined from its initial state by the conservation of total momentum and total energy. This is true irrespective of the exact nature of the forces involved in ...
Bohmian Mechanics
... quantum mechanics, it is probably because they think that, contrary to the official doctrine, physical systems do have quantitative properties (like energy, momentum, spin, etc.) and that properly designed experiments reveal their numerical values. In that view, let us call it the naive one, the mea ...
... quantum mechanics, it is probably because they think that, contrary to the official doctrine, physical systems do have quantitative properties (like energy, momentum, spin, etc.) and that properly designed experiments reveal their numerical values. In that view, let us call it the naive one, the mea ...
A Brief History of Modern Physics and the development of the
... and all of chemistry". It is probably the most important equation of the 20th Century. Its effect on technological progress has been much, much greater than the more famous equation E = mc2. Schrödinger was quite puzzled by the nature of the wave function. What is the physical meaning of Ψ(x,t)? He ...
... and all of chemistry". It is probably the most important equation of the 20th Century. Its effect on technological progress has been much, much greater than the more famous equation E = mc2. Schrödinger was quite puzzled by the nature of the wave function. What is the physical meaning of Ψ(x,t)? He ...
Renormalization group

In theoretical physics, the renormalization group (RG) refers to a mathematical apparatus that allows systematic investigation of the changes of a physical system as viewed at different distance scales. In particle physics, it reflects the changes in the underlying force laws (codified in a quantum field theory) as the energy scale at which physical processes occur varies, energy/momentum and resolution distance scales being effectively conjugate under the uncertainty principle (cf. Compton wavelength).A change in scale is called a ""scale transformation"". The renormalization group is intimately related to ""scale invariance"" and ""conformal invariance"", symmetries in which a system appears the same at all scales (so-called self-similarity). (However, note that scale transformations are included in conformal transformations, in general: the latter including additional symmetry generators associated with special conformal transformations.)As the scale varies, it is as if one is changing the magnifying power of a notional microscope viewing the system. In so-called renormalizable theories, the system at one scale will generally be seen to consist of self-similar copies of itself when viewed at a smaller scale, with different parameters describing the components of the system. The components, or fundamental variables, may relate to atoms, elementary particles, atomic spins, etc. The parameters of the theory typically describe the interactions of the components. These may be variable ""couplings"" which measure the strength of various forces, or mass parameters themselves. The components themselves may appear to be composed of more of the self-same components as one goes to shorter distances.For example, in quantum electrodynamics (QED), an electron appears to be composed of electrons, positrons (anti-electrons) and photons, as one views it at higher resolution, at very short distances. The electron at such short distances has a slightly different electric charge than does the ""dressed electron"" seen at large distances, and this change, or ""running,"" in the value of the electric charge is determined by the renormalization group equation.