Chapter Five
... To accomplish this, we put coefficients in front of the chemical formulas whose atom numbers we wish to increase. Note that you may never change the subscripts already in place in a chemical formula! { Why? ...
... To accomplish this, we put coefficients in front of the chemical formulas whose atom numbers we wish to increase. Note that you may never change the subscripts already in place in a chemical formula! { Why? ...
Additional Review
... Alchemy [1500 AD] In the 1500’s many scientists were________________________________________________ ________________________________________________________________________________________ While they were not able to create gold they did discover many useful properties of matter such as: o density ...
... Alchemy [1500 AD] In the 1500’s many scientists were________________________________________________ ________________________________________________________________________________________ While they were not able to create gold they did discover many useful properties of matter such as: o density ...
Physical and Chemical change: Introduction
... There are some important things to remember about physical changes in matter: ...
... There are some important things to remember about physical changes in matter: ...
Chemical Formulas and Chemical Compounds
... a. How many atoms are represented by the formula Ca(HSO4)2? b. How many moles of oxygen atoms are in a 0.50 mol sample of this ...
... a. How many atoms are represented by the formula Ca(HSO4)2? b. How many moles of oxygen atoms are in a 0.50 mol sample of this ...
Section 1 The Nature of Chemical Reactions
... Classifying Reactions, continued • A single-displacement reaction is a reaction in which one element or radical takes the place of another element or radical in the compound. • Single-displacement reactions have the following general form: AX + B → BX + A • Example: The single-displacement reaction ...
... Classifying Reactions, continued • A single-displacement reaction is a reaction in which one element or radical takes the place of another element or radical in the compound. • Single-displacement reactions have the following general form: AX + B → BX + A • Example: The single-displacement reaction ...
chemical reaction
... • ________ energy is required to ________ the bonds in the reactants than is released by the formation of the ...
... • ________ energy is required to ________ the bonds in the reactants than is released by the formation of the ...
Chemistry - Bourbon County Schools
... and gases at the atomic and molecular levels Use the kinetic molecular theory to explain the states and properties (i.e., microscopic and macroscopic) of matter and phase changes Explain the basis and importance of the absolute temperature scale and convert between the Kelvin and Celsius scales Use ...
... and gases at the atomic and molecular levels Use the kinetic molecular theory to explain the states and properties (i.e., microscopic and macroscopic) of matter and phase changes Explain the basis and importance of the absolute temperature scale and convert between the Kelvin and Celsius scales Use ...
Ab Initio Quantum Chemistry: Thermochemistry and Kinetics
... We can use these tools effectively to teach the chemical principles our students will need. • Build on students’ chemistry education and their prior use of properties to solve problems. • Refresh their recognitions of molecule types using ...
... We can use these tools effectively to teach the chemical principles our students will need. • Build on students’ chemistry education and their prior use of properties to solve problems. • Refresh their recognitions of molecule types using ...
The masses of reactants and products are equal.
... where does their extra mass come from? At one time, scientists thought that chemical reactions could create or destroy matter. During the 1780s the French chemist Antoine Lavoisier (luh-VWAHzee-ay) showed that matter can never be created or destroyed in a chemical reaction. Lavoisier emphasized the ...
... where does their extra mass come from? At one time, scientists thought that chemical reactions could create or destroy matter. During the 1780s the French chemist Antoine Lavoisier (luh-VWAHzee-ay) showed that matter can never be created or destroyed in a chemical reaction. Lavoisier emphasized the ...
Matter - cloudfront.net
... olive oil (other neutral flavored oils will work fine, but avoid extra virgin olive oil as the flavor can be overpowering. Season with salt and pepper to taste. ...
... olive oil (other neutral flavored oils will work fine, but avoid extra virgin olive oil as the flavor can be overpowering. Season with salt and pepper to taste. ...
Teacher Background - Online Learning Exchange
... representative particles: the smallest unit into which a substance can be broken down without a change in composition; usually atoms, molecules, or ions ...
... representative particles: the smallest unit into which a substance can be broken down without a change in composition; usually atoms, molecules, or ions ...
Name __KEY____________ Per. ______ Polarity and
... One specific type of double substitution reaction is combustion where some type of hydrocarbon fuel reacts with oxygen gas and burns. When there is sufficient oxygen present, ___ complete___ (complete/ incomplete) combustion occurs and the products are carbon dioxide and water vapor. When there is n ...
... One specific type of double substitution reaction is combustion where some type of hydrocarbon fuel reacts with oxygen gas and burns. When there is sufficient oxygen present, ___ complete___ (complete/ incomplete) combustion occurs and the products are carbon dioxide and water vapor. When there is n ...
Chemical Reactions and Equations
... 3. Displacement reaction 4. Double displacement reactions 5. Oxidation and Reduction reactions What are ‘Combination Reactions’? When two or more substances (elements or compounds) combine to form a single product, the reactions are called ‘Combination Reactions’. Generally, Combination Reactions ar ...
... 3. Displacement reaction 4. Double displacement reactions 5. Oxidation and Reduction reactions What are ‘Combination Reactions’? When two or more substances (elements or compounds) combine to form a single product, the reactions are called ‘Combination Reactions’. Generally, Combination Reactions ar ...
Chemical Reactions and Equations
... What is a ‘Reaction’? Reaction is a term used for depicting a change or transformation in which a substance decomposes, combines with other substances, or interchanges constituents with other substances. What is a ‘Chemical Reaction’? A chemical change is always accompanied by a chemical reaction. a ...
... What is a ‘Reaction’? Reaction is a term used for depicting a change or transformation in which a substance decomposes, combines with other substances, or interchanges constituents with other substances. What is a ‘Chemical Reaction’? A chemical change is always accompanied by a chemical reaction. a ...
LESSON 23: Exploding Bags
... the structure or composition of the materials change. Chemical reactions occur around us all the time. When a chemical change is complete, the resulting substance(s) is/are different from the original substance(s). The substance or substances that start a chemical reaction are called reactants. The ...
... the structure or composition of the materials change. Chemical reactions occur around us all the time. When a chemical change is complete, the resulting substance(s) is/are different from the original substance(s). The substance or substances that start a chemical reaction are called reactants. The ...
Writing Chemical Formulas and Chemical Reactions
... All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired number (coefficient ...
... All chemical equations must be balanced so that they are consistent with the Law of Conservation of Mass. Here are some suggestions for balancing equations: 1. When balancing equations, always start with the “ugliest” molecule first (polyatomics). 2. To balance, place the desired number (coefficient ...
L2004-01A
... lakes and seas across the world. The carp-shaped robots, costing 20,000 pounds ($29,000) apiece, mimic the movement of real fish and are equipped with chemical sensors to sniff out potentially hazardous pollutants, such as leaks from vessels or underwater pipelines. They will transmit the informatio ...
... lakes and seas across the world. The carp-shaped robots, costing 20,000 pounds ($29,000) apiece, mimic the movement of real fish and are equipped with chemical sensors to sniff out potentially hazardous pollutants, such as leaks from vessels or underwater pipelines. They will transmit the informatio ...
Balancing a Chemical Equation
... • As reactants are converted to products, the bonds holding the atoms together are broken, and new bonds are formed. ...
... • As reactants are converted to products, the bonds holding the atoms together are broken, and new bonds are formed. ...
Comparison of 2008 to 2000 SCH3U_ud
... compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all of which can endanger the health and safety of local populations. Sample questions: What are some chemical reactions used in the manufacture of paper? How might the reactants or products of ...
... compounds can be released into the environment, including cadmium, arsenic, sulphur dioxide, and mercury, all of which can endanger the health and safety of local populations. Sample questions: What are some chemical reactions used in the manufacture of paper? How might the reactants or products of ...
Block 1 - cloudfront.net
... • Number of wheels = ? wheels The desired conversion is tricycles (FSW3HP,) wheels (W). The balanced equation tells you that each tricycle has three wheels, or 1 FSW3HP2 = 3 W. The problem can be solved by using the proper conversion factor derived from this expression. Calculate Solve for the unk ...
... • Number of wheels = ? wheels The desired conversion is tricycles (FSW3HP,) wheels (W). The balanced equation tells you that each tricycle has three wheels, or 1 FSW3HP2 = 3 W. The problem can be solved by using the proper conversion factor derived from this expression. Calculate Solve for the unk ...
PHYSICAL PROPERTIES - can observe w/o changing the
... CHEMICAL CHANGES – alter the substance’s identity at an atomic level. Can’t be reversed with a physical change. Examples: burning, dissolving something in an acid, letting iron rust, letting silver tarnish, mixing vinegar and baking soda, cooking an egg Also called a CHEMICAL REACTION (5 signs to wa ...
... CHEMICAL CHANGES – alter the substance’s identity at an atomic level. Can’t be reversed with a physical change. Examples: burning, dissolving something in an acid, letting iron rust, letting silver tarnish, mixing vinegar and baking soda, cooking an egg Also called a CHEMICAL REACTION (5 signs to wa ...
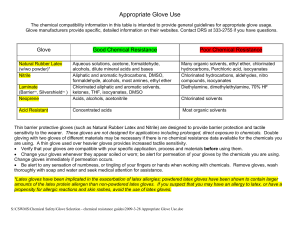
specifications - Omkar Chemicals
... This document is intended only as a guide to the appropriate precautionary handling of material by a person taimed in chemical handling. The user is responsible for a determine the precautions & dangers of this chemical for his or her particular application depending on usage. Adequate protective cl ...
... This document is intended only as a guide to the appropriate precautionary handling of material by a person taimed in chemical handling. The user is responsible for a determine the precautions & dangers of this chemical for his or her particular application depending on usage. Adequate protective cl ...
Chapter 1: Quiz Review - Wetaskiwin Composite High School
... 4. A binary ionic compound is formed from elements A and B. The formula is A2B. What inference can you make about this substance from the formula. A. A is a metal and B is a non-metal C. B is a metal and A is a non-metal B. The name will contain the prefixes di and mono D. The compound contains a po ...
... 4. A binary ionic compound is formed from elements A and B. The formula is A2B. What inference can you make about this substance from the formula. A. A is a metal and B is a non-metal C. B is a metal and A is a non-metal B. The name will contain the prefixes di and mono D. The compound contains a po ...
Chemical Corps

The Chemical Corps is the branch of the United States Army tasked with defending against chemical, biological, radiological, and nuclear (CBRN) weapons. The corps was founded as the Chemical Warfare Service (CWS) during World War I. Its name was changed to the Chemical Corps in 1946.