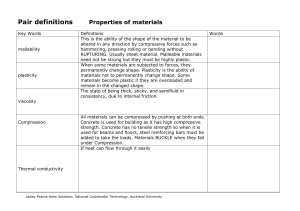
AP Chemistry
... 6.5.3.2 subshell = the set of orbitals that have the same n and ℓ values 6.5.3.3 The shell with principal quantum number n will consist of n subshells 6.5.3.4 Each subshell has specific number of orbitals 6.5.3.4.1 s orbitals are singlets; 6.5.3.4.2 p orbitals come in sets of 3; 6.5.3.4.3 d orbitals ...
... 6.5.3.2 subshell = the set of orbitals that have the same n and ℓ values 6.5.3.3 The shell with principal quantum number n will consist of n subshells 6.5.3.4 Each subshell has specific number of orbitals 6.5.3.4.1 s orbitals are singlets; 6.5.3.4.2 p orbitals come in sets of 3; 6.5.3.4.3 d orbitals ...
Calculation of state selective field ionization of hydrogen atoms in a
... increased from the value where it could have decayed. The second effect is that the magnetic field can add other relevant time scales to the system so that the rapidity of the ramp can cause the electron to transfer energy between different types of motion. Quantum mechanics adds other time dependen ...
... increased from the value where it could have decayed. The second effect is that the magnetic field can add other relevant time scales to the system so that the rapidity of the ramp can cause the electron to transfer energy between different types of motion. Quantum mechanics adds other time dependen ...
Induced EMF - Edvantage Science
... Magnetism, EMF, and Electric Current An Englishman, Michael Faraday (1791-1867) and an American, Joseph Henry (17971878), working independently discovered that magnetism could produce or induce a current in a circuit. Inducing an EMF in a Straight Piece of Wire A current in circuit can be induced if ...
... Magnetism, EMF, and Electric Current An Englishman, Michael Faraday (1791-1867) and an American, Joseph Henry (17971878), working independently discovered that magnetism could produce or induce a current in a circuit. Inducing an EMF in a Straight Piece of Wire A current in circuit can be induced if ...
Transparencies - Rencontres de Moriond
... A. Chou, J. Steffen, A. Uphadye, A.W. and W. Wester “ [Photon]-[dilaton-like chameleon particle] regeneration using a "particle trapped in a jar" technique “ - http://gammev.fnal.gov ...
... A. Chou, J. Steffen, A. Uphadye, A.W. and W. Wester “ [Photon]-[dilaton-like chameleon particle] regeneration using a "particle trapped in a jar" technique “ - http://gammev.fnal.gov ...
LEP 5.1.08 Atomic spectra of two-electron systems: He, Hg
... 1. Determination of the wavelengths of the most intense spectral lines of He. 2. Determination of the wavelengths of the most intense spectral lines of Hg. Set-up and procedure The experimental set-up is shown in Fig. 1. Helium or mercury spectral tubes connected to the high voltage power supply uni ...
... 1. Determination of the wavelengths of the most intense spectral lines of He. 2. Determination of the wavelengths of the most intense spectral lines of Hg. Set-up and procedure The experimental set-up is shown in Fig. 1. Helium or mercury spectral tubes connected to the high voltage power supply uni ...
Chem 1a Review
... noble gases because they have the highest number of protons (positive charge) for that quantum number n. B: Exception to general trend of increase Iz with increase Z. Due to going from filling 1s shell to 1p shell and since p penetrates less well then s it is easier to remove. C: Exception to genera ...
... noble gases because they have the highest number of protons (positive charge) for that quantum number n. B: Exception to general trend of increase Iz with increase Z. Due to going from filling 1s shell to 1p shell and since p penetrates less well then s it is easier to remove. C: Exception to genera ...
Monday, Nov. 12, 2012
... • We must use wave functions to calculate the probability distributions of the electrons. • The “position” of the electron is spread over space and is not well defined. • We may use the radial wave function R(r) to calculate radial probability distributions of the electron. • The probability of find ...
... • We must use wave functions to calculate the probability distributions of the electrons. • The “position” of the electron is spread over space and is not well defined. • We may use the radial wave function R(r) to calculate radial probability distributions of the electron. • The probability of find ...
Chapter Excerpt
... Of Ice Cold Beer.” These molecules are attracted to one another using weak London dispersion forces (see Skill 1.3d). ...
... Of Ice Cold Beer.” These molecules are attracted to one another using weak London dispersion forces (see Skill 1.3d). ...
Introduction to RF Cavities for Accelerators
... Wakefields are only induced by the longitudinal electric field so dipole wakes are only induced by off-axis bunches. Once induced the dipole wakes can apply a kick via the transverse fields so on-axis bunches can still experience the effect of the wakes from preceding bunches. ...
... Wakefields are only induced by the longitudinal electric field so dipole wakes are only induced by off-axis bunches. Once induced the dipole wakes can apply a kick via the transverse fields so on-axis bunches can still experience the effect of the wakes from preceding bunches. ...
Electronic Structure of Multi-Electron Quantum Dots
... plus the pairwise Coulomb interactions. The fact that we are working in a solid state media is modelled by using the relative effective mass m* and the effective ...
... plus the pairwise Coulomb interactions. The fact that we are working in a solid state media is modelled by using the relative effective mass m* and the effective ...
APS104H1_20161_661461623642Lecture 2
... atom. Therefore, Heisenberg said that we shouldn't view electrons as moving in well-defined orbits about the nucleus! With Heisenberg's uncertainty principle in mind, an Austrian physicist named Erwin Schrodinger derived a set of equations or wave functions (Ψ) in 1926 for electrons. According to Sc ...
... atom. Therefore, Heisenberg said that we shouldn't view electrons as moving in well-defined orbits about the nucleus! With Heisenberg's uncertainty principle in mind, an Austrian physicist named Erwin Schrodinger derived a set of equations or wave functions (Ψ) in 1926 for electrons. According to Sc ...
Periodic Properties of the Elements
... Magnetic Properties • Although an electron behaves like a tiny magnet, two electrons that are opposite in spin cancel each other. Only atoms with unpaired electrons exhibit magnetic susceptibility (see Fig. 8.2). – A paramagnetic substance is one that is weakly attracted by a magnetic field, usuall ...
... Magnetic Properties • Although an electron behaves like a tiny magnet, two electrons that are opposite in spin cancel each other. Only atoms with unpaired electrons exhibit magnetic susceptibility (see Fig. 8.2). – A paramagnetic substance is one that is weakly attracted by a magnetic field, usuall ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.