Lecture 18 - UConn Physics
... shown in Figure below. The projectile passes through two coils separated by a distance d. As the projectile passes through each coil a pulse of emf is induced in the coil. The time interval between pulses can be measured accurately with an oscilloscope, and thus the speed can be determined. (a) Sket ...
... shown in Figure below. The projectile passes through two coils separated by a distance d. As the projectile passes through each coil a pulse of emf is induced in the coil. The time interval between pulses can be measured accurately with an oscilloscope, and thus the speed can be determined. (a) Sket ...
Ch. 13-14 Review
... if n = 1, then ℓ can be 0 (s orbital) = 1 sublevel if n = 4, then ℓ can be 0 (s), 1(p), 2(d), 3(f) = 4 sublevels Magnetic quantum number (ml) ...
... if n = 1, then ℓ can be 0 (s orbital) = 1 sublevel if n = 4, then ℓ can be 0 (s), 1(p), 2(d), 3(f) = 4 sublevels Magnetic quantum number (ml) ...
Multi-electron Atoms
... Atoms with Z>1 contain >1 electron. This changes the atomic structure considerably because in addition to the electron-nucleus interaction, there is the repulsive electron-electron interaction. Calculations show that allowed electron energies are no longer solely determined by the single quantum num ...
... Atoms with Z>1 contain >1 electron. This changes the atomic structure considerably because in addition to the electron-nucleus interaction, there is the repulsive electron-electron interaction. Calculations show that allowed electron energies are no longer solely determined by the single quantum num ...
Electron Configuration
... Pauli Exclusion Principle •Spin is a quantum mechanical property of electrons and may be thought of as clockwise or counterclockwise. •A vertical arrow indicates an electron and its direction of spin ( or ). •An orbital containing paired electrons is written as . ...
... Pauli Exclusion Principle •Spin is a quantum mechanical property of electrons and may be thought of as clockwise or counterclockwise. •A vertical arrow indicates an electron and its direction of spin ( or ). •An orbital containing paired electrons is written as . ...
Magnetic-field-induced Anderson localization in a strongly
... major topic in condensed matter physics. An important aspect of this subject has been the physics of the so-called "weak localization, which describes localization as due between electronic paths and interferences to constructive their time-reversed counterparts [I]. This picture holds at finite tem ...
... major topic in condensed matter physics. An important aspect of this subject has been the physics of the so-called "weak localization, which describes localization as due between electronic paths and interferences to constructive their time-reversed counterparts [I]. This picture holds at finite tem ...
Modern Physics 342
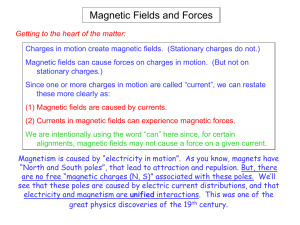
... Two opposite dipoles in the same non-uniform electric field are affected by opposite net forces that lead to displacing each dipole up and down according to their respective alignments. ...
... Two opposite dipoles in the same non-uniform electric field are affected by opposite net forces that lead to displacing each dipole up and down according to their respective alignments. ...
Effects of Toxic Materials
... Magnetic Seperation-the ore is passed over a drum inside which there is a magnet which is stationery. The magnetic material will be carried on further than the non-magnetic and therefore will be seperated. Hydrometallurgy- uses aqueous solutions called leaches to serperate metals from ...
... Magnetic Seperation-the ore is passed over a drum inside which there is a magnet which is stationery. The magnetic material will be carried on further than the non-magnetic and therefore will be seperated. Hydrometallurgy- uses aqueous solutions called leaches to serperate metals from ...
The Quantum Numbers
... It is possible the electrons spin in opposite directions and therefore, produce opposite magnetic fields that attract rather than repel one another. Scientist refer to these possible spins as (+1/2) and (-1/2). The fact that each electron in an orbital must have different spin quantum numbers led Wo ...
... It is possible the electrons spin in opposite directions and therefore, produce opposite magnetic fields that attract rather than repel one another. Scientist refer to these possible spins as (+1/2) and (-1/2). The fact that each electron in an orbital must have different spin quantum numbers led Wo ...
Divergence and Curl of the Magnetic Field
... where the surface S over which we integrate on the last line is the boundary of the volume V over which we integrate in the Biot–Savart–Laplace equation (9). This volume must include everywhere the current flows, but we may just as well use a bigger volume. Indeed, let’s take a bigger volume V, so n ...
... where the surface S over which we integrate on the last line is the boundary of the volume V over which we integrate in the Biot–Savart–Laplace equation (9). This volume must include everywhere the current flows, but we may just as well use a bigger volume. Indeed, let’s take a bigger volume V, so n ...
Magnetic Fields and Electric Currents
... • Electric current in the second wire was made only when the magnetic field was changing. ...
... • Electric current in the second wire was made only when the magnetic field was changing. ...
Magnetic Resonance Imaging (MRI)
... We have seen in SNI how the existence of different electronic energy in atoms leads to line spectra. Careful experiments showed, surprisingly, that some of these lines are split, with differences in wavelength that are much smaller than could be explained by any known differences in the atomic energ ...
... We have seen in SNI how the existence of different electronic energy in atoms leads to line spectra. Careful experiments showed, surprisingly, that some of these lines are split, with differences in wavelength that are much smaller than could be explained by any known differences in the atomic energ ...
L08_Magnetic_Field
... But magnetism was known and applied from ancient times… (From Wikipedia) Lodestone refers to either: (1) Magnetite, a magnetic mineral form of Fe3O4, one of several iron oxides. (2) A piece of intensely magnetic magnetite that was used as an early form of magnetic compass. Iron, steel and ordinary ...
... But magnetism was known and applied from ancient times… (From Wikipedia) Lodestone refers to either: (1) Magnetite, a magnetic mineral form of Fe3O4, one of several iron oxides. (2) A piece of intensely magnetic magnetite that was used as an early form of magnetic compass. Iron, steel and ordinary ...
Quantum Theory - developed by German physicist Max Planck
... ("L" will be used to replace °T’ to better differentiate the letter from the number1) There are 4 quantum numbers that can describe the distribution of electrons: 1 - ~antum number (n) describes the average distance the electron is from the nucleus of the atom (energy levels or shells) **’~n values ...
... ("L" will be used to replace °T’ to better differentiate the letter from the number1) There are 4 quantum numbers that can describe the distribution of electrons: 1 - ~antum number (n) describes the average distance the electron is from the nucleus of the atom (energy levels or shells) **’~n values ...
eprint_11_10723_328
... the right hand grip rule), with a magnitude equal to the current in the loop times the area of the loop. In addition to current loops, the electron, among other fundamental particles, has a magnetic dipole moment. This is because it generates a magnetic field that is identical to that generated by a ...
... the right hand grip rule), with a magnitude equal to the current in the loop times the area of the loop. In addition to current loops, the electron, among other fundamental particles, has a magnetic dipole moment. This is because it generates a magnetic field that is identical to that generated by a ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.