atomsagain
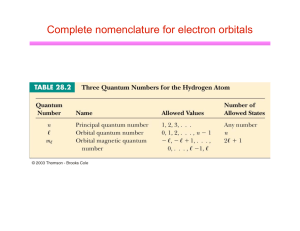
... To describe one electron, we give the following information: •n – this is called the shell Shells are labeled by numbers 1, 2, 3, . . . •l – this is called the subshell Subshells are denoted by letter s, p, d, f, g •m – this is called the orbital orbital depends on choice of axes •ms – this is ...
... To describe one electron, we give the following information: •n – this is called the shell Shells are labeled by numbers 1, 2, 3, . . . •l – this is called the subshell Subshells are denoted by letter s, p, d, f, g •m – this is called the orbital orbital depends on choice of axes •ms – this is ...
magnetic field
... produce a magnetic field • Permanent magnet. Elementary particles such as electrons have an intrinsic magnetic field around them. The magnetic fields of the electrons in certain materials add together to give a net magnetic field around the material. Such addition is the reason why a permanent magne ...
... produce a magnetic field • Permanent magnet. Elementary particles such as electrons have an intrinsic magnetic field around them. The magnetic fields of the electrons in certain materials add together to give a net magnetic field around the material. Such addition is the reason why a permanent magne ...
is the “quantum number”
... quantum numbers also result in small energy differences • Pauli exclusion principle: no two electrons in the same atom can be in the same quantum state • Electrons are grouped into shells and subshells • Periodic table reflects shell structure Atoms with the same number of electrons in their outer s ...
... quantum numbers also result in small energy differences • Pauli exclusion principle: no two electrons in the same atom can be in the same quantum state • Electrons are grouped into shells and subshells • Periodic table reflects shell structure Atoms with the same number of electrons in their outer s ...
Complete nomenclature for electron orbitals
... time l Electron is not confined to any particular orbital distance from the nucleus but has a probability of being at various distances (with a maximum probability at the Bohr radius ao) l Think of the electron as being in an electron cloud ...
... time l Electron is not confined to any particular orbital distance from the nucleus but has a probability of being at various distances (with a maximum probability at the Bohr radius ao) l Think of the electron as being in an electron cloud ...
26.2 Magnetic field
... When a piece of magnetic material is brought close to the pole of a permanent magnet, it is __________________ and behaves like a temporary magnet. ...
... When a piece of magnetic material is brought close to the pole of a permanent magnet, it is __________________ and behaves like a temporary magnet. ...
Electrons in Atoms
... s, electrons are placed in individual orbitals before they are paired up. • Electrons fill like people do on a bus. You would never sit right next to someone you did not know if there are free seats available, unless of course all the seats are taken then you must pair up. ...
... s, electrons are placed in individual orbitals before they are paired up. • Electrons fill like people do on a bus. You would never sit right next to someone you did not know if there are free seats available, unless of course all the seats are taken then you must pair up. ...
lecture31
... There are four different quantum numbers needed to specify the state of an electron in an atom: 1) Principal quantum number n gives the total energy: ...
... There are four different quantum numbers needed to specify the state of an electron in an atom: 1) Principal quantum number n gives the total energy: ...
zeeman effect
... The angles 1 and 2 can be calculated from the scalar products of the respective vectors: Lˆ Jˆ | L || J | cos1 Sˆ Jˆ | S || J | cos ...
... The angles 1 and 2 can be calculated from the scalar products of the respective vectors: Lˆ Jˆ | L || J | cos1 Sˆ Jˆ | S || J | cos ...
Solar System Foldable Checklist
... _____Relative size (compare to Earth) _____Number of moons (name of largest) _____Surface features (temperature) _____Type of atmosphere _____Current missions to planet (explorations) _____ Magnetic field _____ Internal structure _____Unique facts Neptune _____Relative size (compare to Earth) _____N ...
... _____Relative size (compare to Earth) _____Number of moons (name of largest) _____Surface features (temperature) _____Type of atmosphere _____Current missions to planet (explorations) _____ Magnetic field _____ Internal structure _____Unique facts Neptune _____Relative size (compare to Earth) _____N ...
CHM1045 - Michael Blaber
... 1. A negatively charged particle is moving towards you through a magnetic field and is deflected downward (as shown in the following diagram). Based upon the direction of deflection, and the charge of the particle, write in the appropriate pole (i.e. "N" or "S") on the two magnets to indicate the di ...
... 1. A negatively charged particle is moving towards you through a magnetic field and is deflected downward (as shown in the following diagram). Based upon the direction of deflection, and the charge of the particle, write in the appropriate pole (i.e. "N" or "S") on the two magnets to indicate the di ...
magnet - UniMAP Portal
... saturation. (The value of B at point B on the hysteresis curve.) • Coercive Force - The amount of reverse magnetic field which must be applied to a magnetic material to make the magnetic flux return to zero. (The value of H at point C on the hysteresis curve.) ...
... saturation. (The value of B at point B on the hysteresis curve.) • Coercive Force - The amount of reverse magnetic field which must be applied to a magnetic material to make the magnetic flux return to zero. (The value of H at point C on the hysteresis curve.) ...
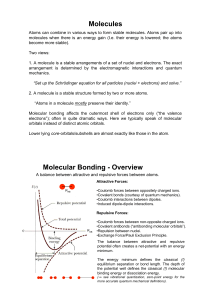
Molecules Molecular Bonding
... •Exchange Force/Pauli Exclusion Principle. The balance between attractive and repulsive potential often creates a net-potential with an energy minimum. The energy minimum defines the classical (!) equilibrium separation or bond length. The depth of the potential well defines the classical (!) molecu ...
... •Exchange Force/Pauli Exclusion Principle. The balance between attractive and repulsive potential often creates a net-potential with an energy minimum. The energy minimum defines the classical (!) equilibrium separation or bond length. The depth of the potential well defines the classical (!) molecu ...
INORGANIC CHEMISTRY F R O N T I E R S
... time from this source (for details, see ESI†).22 Note that this approximation neglects other decoherence sources; therefore, it only provides an upper bound for the decoherence time. Nevertheless, it is a useful tool to provide a starting point towards an inexpensive theoretical quantification of de ...
... time from this source (for details, see ESI†).22 Note that this approximation neglects other decoherence sources; therefore, it only provides an upper bound for the decoherence time. Nevertheless, it is a useful tool to provide a starting point towards an inexpensive theoretical quantification of de ...
Electromagnetic Induction
... Steps in problem solving – Lenz’s Law 3. Use the right hand rule-1 to find the direction of the induced current 4. Always keep in mind that there are two magnetic fields a. An external field whose flux must be changed if it is to induce an electric current b. A magnetic field produced by the induced ...
... Steps in problem solving – Lenz’s Law 3. Use the right hand rule-1 to find the direction of the induced current 4. Always keep in mind that there are two magnetic fields a. An external field whose flux must be changed if it is to induce an electric current b. A magnetic field produced by the induced ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.