Microsoft Word Format - University of Toronto Physics
... As was first suggested by Goudsmit and Uhlenbeck, all electrons have an intrinsic angular momentum which may be attributed to a spin about an internal axis. Associated with this spin is a magnetic dipole moment. In most substances, the orbital angular momenta and the spin angular momenta of the elec ...
... As was first suggested by Goudsmit and Uhlenbeck, all electrons have an intrinsic angular momentum which may be attributed to a spin about an internal axis. Associated with this spin is a magnetic dipole moment. In most substances, the orbital angular momenta and the spin angular momenta of the elec ...
The Stern Gerlach Experiment Abstract
... moments J and I causes a rapid precession about the total angular momentum axis, F. The quantum number of the sum is f = i ± j = 1 or 2. For each possibility, we associate a different magnetic moment. In our setup, at temperatures of 200◦ C, almost all of the potassium atoms will be in the ground st ...
... moments J and I causes a rapid precession about the total angular momentum axis, F. The quantum number of the sum is f = i ± j = 1 or 2. For each possibility, we associate a different magnetic moment. In our setup, at temperatures of 200◦ C, almost all of the potassium atoms will be in the ground st ...
File
... •All matter is made of atoms. •Atoms have a magnetic fields. Atoms group together when their magnetic fields align. These groups are called domains. ...
... •All matter is made of atoms. •Atoms have a magnetic fields. Atoms group together when their magnetic fields align. These groups are called domains. ...
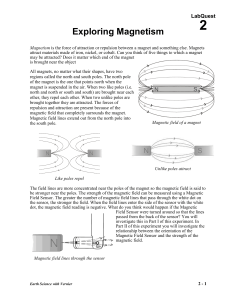
02 Expl Magnet LQ
... Magnetism is the force of attraction or repulsion between a magnet and something else. Magnets attract materials made of iron, nickel, or cobalt. Can you think of five things to which a magnet may be attracted? Does it matter which end of the magnet is brought near the object All magnets, no matter ...
... Magnetism is the force of attraction or repulsion between a magnet and something else. Magnets attract materials made of iron, nickel, or cobalt. Can you think of five things to which a magnet may be attracted? Does it matter which end of the magnet is brought near the object All magnets, no matter ...
St_Pierre_2002 - Scientific and Clinical Applications of Magnetic
... strong fields can saturate magnetization by creating single domain ...
... strong fields can saturate magnetization by creating single domain ...
The Quantum Hall Effect in Graphene
... the charges experience the Lorenz force and are deflected to one side of the conductor. Then, equal but opposite charges accumulate on the opposite side. The result is an asymmetric distribution of charge carriers on the conductor’s surface. This separation of charges establishes an electric field t ...
... the charges experience the Lorenz force and are deflected to one side of the conductor. Then, equal but opposite charges accumulate on the opposite side. The result is an asymmetric distribution of charge carriers on the conductor’s surface. This separation of charges establishes an electric field t ...
Modern Model of the Atom
... Find the noble gas that comes before the element and is numerically the closest Write its symbol in brackets Write the remainder of the electron ...
... Find the noble gas that comes before the element and is numerically the closest Write its symbol in brackets Write the remainder of the electron ...
Atomic Structure Atomic Structure
... noble gas ! outer electrons: after that highest energy levels ! valence electrons: same as outer, involved in forming chemical bonds ...
... noble gas ! outer electrons: after that highest energy levels ! valence electrons: same as outer, involved in forming chemical bonds ...
gfgf-odt - Ranjit Tutorials
... Students must not use calculators and any other unfair means while taking the test. The duration of the test is 30 minutes. You will not be able to submit the test after the time is over. There will be total 15 MCQ in this test. The test will consist of only objective type multiple choice questions ...
... Students must not use calculators and any other unfair means while taking the test. The duration of the test is 30 minutes. You will not be able to submit the test after the time is over. There will be total 15 MCQ in this test. The test will consist of only objective type multiple choice questions ...
Ballistic Transport in a two-dimensional Electron System
... nearby the GaAs/AlGaAs interface. This is called a two-dimensional electron system (2DES). These electrons see only a small disturbing coulomb potential due to the ionized Si atoms far away from the conducting layer. Consequently they are able to travel typically some µm without being scattered. The ...
... nearby the GaAs/AlGaAs interface. This is called a two-dimensional electron system (2DES). These electrons see only a small disturbing coulomb potential due to the ionized Si atoms far away from the conducting layer. Consequently they are able to travel typically some µm without being scattered. The ...
QuantumDots
... symmetric spatial wave function and an anti symmetric spin (Coulomb dominates): |ψs> ~ (|12> + |21>) (|↓↑> - |↑↓>) • The triplet states are: ...
... symmetric spatial wave function and an anti symmetric spin (Coulomb dominates): |ψs> ~ (|12> + |21>) (|↓↑> - |↑↓>) • The triplet states are: ...
Section 4-2 The Quantum Model of the Atom Problems with the Bohr
... I. This equation defined all of the locations in an atom where electrons could and could not exist. II. Combined with the uncertainty principle, the wave equation defined areas of probability of electron location III.The “wave functions” of particular electrons are 3D regions of space that the elect ...
... I. This equation defined all of the locations in an atom where electrons could and could not exist. II. Combined with the uncertainty principle, the wave equation defined areas of probability of electron location III.The “wave functions” of particular electrons are 3D regions of space that the elect ...
ppt
... can have the same 4 quantum numbers. Electrons in the same orbital can’t have the same spin Hund’s Rule: One electron occupies each of sub-orbitals in the same energy level before a second can occupy the same sub-orbital Aufbau Principle: each electron is added to the lowest energy orbital avail ...
... can have the same 4 quantum numbers. Electrons in the same orbital can’t have the same spin Hund’s Rule: One electron occupies each of sub-orbitals in the same energy level before a second can occupy the same sub-orbital Aufbau Principle: each electron is added to the lowest energy orbital avail ...
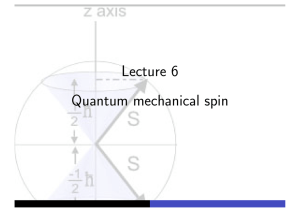
Quantum mechanical spin - Theory of Condensed Matter
... In experiment, a beam of silver atoms were passed through inhomogeneous magnetic field and collected on photographic plate. Since silver involves spherically symmetric charge distribution plus one 5s electron, total angular momentum of ground state has L = 0. If outer electron in 5p state, L = 1 and ...
... In experiment, a beam of silver atoms were passed through inhomogeneous magnetic field and collected on photographic plate. Since silver involves spherically symmetric charge distribution plus one 5s electron, total angular momentum of ground state has L = 0. If outer electron in 5p state, L = 1 and ...
The study of biology can help you better understand
... Write noble gas notation for electrons configuration for the following atoms: d orbital can hold maximum 10 electrons. K ________________________________________________________________ Ca ________________________________________________________________ ...
... Write noble gas notation for electrons configuration for the following atoms: d orbital can hold maximum 10 electrons. K ________________________________________________________________ Ca ________________________________________________________________ ...
... and relatively sharp interfaces. In the last several years, there has been a considerable interest in quasi-zero dimensional self-assembled quantum dots (QDs), formed through the Stranki-Krastanow growth mode by deposition a material on the substrate with different lattice parameter [1]. This intere ...
F AT is an approximation of T
... For the purposes of modeling we work backwards. Given a certain object, we compute the horizontal (HA) and vertical (ZA) components of the anomaly and combine them to obtain FAT - the anomaly we obtain from the proton precession magnetometer measurements. ...
... For the purposes of modeling we work backwards. Given a certain object, we compute the horizontal (HA) and vertical (ZA) components of the anomaly and combine them to obtain FAT - the anomaly we obtain from the proton precession magnetometer measurements. ...
magnetic circuit
... magnetic flux density B in the core bears a definite ratio to the magnetic field strength H. This ratio is called permeability of free space. Thus, for vacuum or air, ...
... magnetic flux density B in the core bears a definite ratio to the magnetic field strength H. This ratio is called permeability of free space. Thus, for vacuum or air, ...
μ s
... The lack of retraceability shown in the Figure is called hysteresis, and the curve bcdeb is called a hysteresis loop. Note that at points c and e the iron core is magnetized, even though there is no current in the toroid windings; this is the familiar phenomenon of permanent magnetism. Hysteresis ca ...
... The lack of retraceability shown in the Figure is called hysteresis, and the curve bcdeb is called a hysteresis loop. Note that at points c and e the iron core is magnetized, even though there is no current in the toroid windings; this is the familiar phenomenon of permanent magnetism. Hysteresis ca ...
Magnetic Materials Background: 9. Hard Magnets
... maximum in 1956 with the introduction of anisotropic columnar alnico 9, with an energy product of ~80kJm-3. These alloys are still used today as they have a high Curie temperature (~850°C), and as a result can operate at higher temperatures as well as having more stable properties around room temper ...
... maximum in 1956 with the introduction of anisotropic columnar alnico 9, with an energy product of ~80kJm-3. These alloys are still used today as they have a high Curie temperature (~850°C), and as a result can operate at higher temperatures as well as having more stable properties around room temper ...
estimation of subsurface residual stress depth profiles via wideband
... James Thomas, et al. American Stress Technologies, Inc., USA ...
... James Thomas, et al. American Stress Technologies, Inc., USA ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.