Magnetism - APlusPhysics
... b. Use superposition to determine the magnetic field produced by two long wires. c. Calculate the force of attraction or repulsion between two long current-carrying wires. 4. Biot-Savart law and Ampere’s law a. Students should understand the Biot-Savart Law, so they can: i. Deduce the magnitude and ...
... b. Use superposition to determine the magnetic field produced by two long wires. c. Calculate the force of attraction or repulsion between two long current-carrying wires. 4. Biot-Savart law and Ampere’s law a. Students should understand the Biot-Savart Law, so they can: i. Deduce the magnitude and ...
Atomic Physics
... seating of patrons. He will treat tables as sub-shells, and will only seat patrons if the number of the people to be seated adds up to a complete sub-shell. Of the numbers below, the number he would not be willing to seat at one table is a. b. c. d. e. ...
... seating of patrons. He will treat tables as sub-shells, and will only seat patrons if the number of the people to be seated adds up to a complete sub-shell. Of the numbers below, the number he would not be willing to seat at one table is a. b. c. d. e. ...
Magnetism: Models and Mechanisms - cond
... of the crystal field, a magnetic ion in a crystal might lose, totally or partially, its spin, angular or total moment. Or, sometimes, it is the other way around. This happens for Mn3+ ions, which should have a J = 0 ground state according to the third Hund’s rule. However in perovskites such as LaMn ...
... of the crystal field, a magnetic ion in a crystal might lose, totally or partially, its spin, angular or total moment. Or, sometimes, it is the other way around. This happens for Mn3+ ions, which should have a J = 0 ground state according to the third Hund’s rule. However in perovskites such as LaMn ...
Stern-Gerlach - University of Hawaii
... In 1927, two experiments were done by young graduate students in Urbana, Illinois and in Aberdeen, Scotland! R.G.J. Fraser measured the shape of hydrogen atom by scattering and found it to be spherically symmetric; T. E. Phipps and J. B. Taylor did a Stern-Gerlach experiment with hydrogen atoms and ...
... In 1927, two experiments were done by young graduate students in Urbana, Illinois and in Aberdeen, Scotland! R.G.J. Fraser measured the shape of hydrogen atom by scattering and found it to be spherically symmetric; T. E. Phipps and J. B. Taylor did a Stern-Gerlach experiment with hydrogen atoms and ...
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... a. 6, 0, -1, +½ b. 6, 1, 1, +½ c. 6, 0, 0, +½ d. 6, 1, 0, +½ e. 6, 0, 1, -½ ____ 3. Which of the following rules states that no two electrons in an atom can have the same set of quantum numbers? a. Hund’s rule b. The Heisenberg Uncertainty principle c. The Pauli Exclusion principle d. The deBroglie ...
... a. 6, 0, -1, +½ b. 6, 1, 1, +½ c. 6, 0, 0, +½ d. 6, 1, 0, +½ e. 6, 0, 1, -½ ____ 3. Which of the following rules states that no two electrons in an atom can have the same set of quantum numbers? a. Hund’s rule b. The Heisenberg Uncertainty principle c. The Pauli Exclusion principle d. The deBroglie ...
Ferroelectrics
... A naïve picture • The local alignment of dipoles can exist over any length scale. • Different regions may exist with different polarisation orientations: – Call these “domains” in line with magnetic materials. – In contrast with magnetism, domain walls are abrupt. ...
... A naïve picture • The local alignment of dipoles can exist over any length scale. • Different regions may exist with different polarisation orientations: – Call these “domains” in line with magnetic materials. – In contrast with magnetism, domain walls are abrupt. ...
Magnetic field lines
... Magnetic and Electric Fields An electric field surrounds any stationary electric charge A magnetic field surrounds any moving electric charge A magnetic field surrounds any magnetic material ...
... Magnetic and Electric Fields An electric field surrounds any stationary electric charge A magnetic field surrounds any moving electric charge A magnetic field surrounds any magnetic material ...
Record High Single-Ion Magnetic Moments Through 4f 5d1 Electron
... 1), a result that has been observed previously for tris(cyclopentadienyl) complexes.13 These lower values can be ascribed to the size of the crystal field splitting, such that at room temperature the full J manifold is not completely populated. However, for ErIII, TmIII, and YbIII, the χMT values at ...
... 1), a result that has been observed previously for tris(cyclopentadienyl) complexes.13 These lower values can be ascribed to the size of the crystal field splitting, such that at room temperature the full J manifold is not completely populated. However, for ErIII, TmIII, and YbIII, the χMT values at ...
Electron Discovery (PowerPoint)
... charge-to-mass ratio of cathode rays by balancing an upward deflection from an electric field with a downward deflection from a magnetic (resulting in path b). Thomson argued that these “cathode rays” were tiny negatively charged particles and were constituents of atoms, not ions or atoms themselves ...
... charge-to-mass ratio of cathode rays by balancing an upward deflection from an electric field with a downward deflection from a magnetic (resulting in path b). Thomson argued that these “cathode rays” were tiny negatively charged particles and were constituents of atoms, not ions or atoms themselves ...
1. dia
... energy then the energy difference can be emitted as a photon. This may gives a line in the visible spectrum. In the presence of an external magnetic field, these different states will have different energies due to having different orientations of the magnetic dipoles in the external field, so the a ...
... energy then the energy difference can be emitted as a photon. This may gives a line in the visible spectrum. In the presence of an external magnetic field, these different states will have different energies due to having different orientations of the magnetic dipoles in the external field, so the a ...
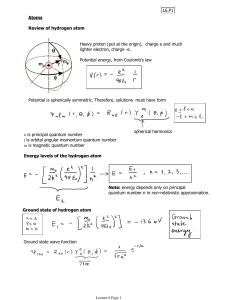
Lecture 6 - physics.udel.edu
... learn how to account for the interaction term later in this course. Now, we need to include spin in our description. Electrons have spin antisymmetric. ...
... learn how to account for the interaction term later in this course. Now, we need to include spin in our description. Electrons have spin antisymmetric. ...
Self-Assembly of Colloidal Pyramids in Magnetic Fields
... pyramids with tails (i.e., with linear chains attached to the apex), but a physical explanation for this phenomenon has not yet been revealed. That is, we do not yet understand why these tails do not conform to increase the size of the pyramid in order to make it more stable. However, it should be e ...
... pyramids with tails (i.e., with linear chains attached to the apex), but a physical explanation for this phenomenon has not yet been revealed. That is, we do not yet understand why these tails do not conform to increase the size of the pyramid in order to make it more stable. However, it should be e ...
Chapter 19: Fermi
... • Each atom in the crystal lattice of the metal is assumed to part with some number of its outer valence electrons, which can then move freely about in the metal. ...
... • Each atom in the crystal lattice of the metal is assumed to part with some number of its outer valence electrons, which can then move freely about in the metal. ...
Specialization: 010700/02 Physics of atoms and molecules
... calculations are performed. In the first only the correlation of the valence electrons is taken into account. In the second the correlation of 5s5p5d core electrons of lead and 1s electrons of fluorine are taken into account as well. The difference between the results of these two series is adjusted ...
... calculations are performed. In the first only the correlation of the valence electrons is taken into account. In the second the correlation of 5s5p5d core electrons of lead and 1s electrons of fluorine are taken into account as well. The difference between the results of these two series is adjusted ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.