A Lattice Model of Liquid Helium» II I. Introduction
... quantitative conclusions on specific heat and pressure, etc., but also qualitative explanation for the last of the three questions proposed at the begining of I. Namely as to the question why liquid helium has negative thermal expansion coefficient just below the J.-point, the previous treatment see ...
... quantitative conclusions on specific heat and pressure, etc., but also qualitative explanation for the last of the three questions proposed at the begining of I. Namely as to the question why liquid helium has negative thermal expansion coefficient just below the J.-point, the previous treatment see ...
Solid - Liquid Phase Diagram of a Binary Mixture: The Question of
... Two components will be assigned for this experiment. One will be a carboxylic acid, and the other will be a relatively non-polar material. Pre-Lab: Access the Computer-Simulated Experiment FPWin.STA or FPMac, and observe the DEMO section of this simulation. If you have any questions about the perfor ...
... Two components will be assigned for this experiment. One will be a carboxylic acid, and the other will be a relatively non-polar material. Pre-Lab: Access the Computer-Simulated Experiment FPWin.STA or FPMac, and observe the DEMO section of this simulation. If you have any questions about the perfor ...
Molar Mass by Freezing Point Depression
... melting point without the formation of crystals - a phenomenon known as “supercooling”. As soon as the first crystals form, however, the temperature rises quickly to the melting point and remains constant. The heat (enthalpy) released by the solidification process exactly compensates the heat transf ...
... melting point without the formation of crystals - a phenomenon known as “supercooling”. As soon as the first crystals form, however, the temperature rises quickly to the melting point and remains constant. The heat (enthalpy) released by the solidification process exactly compensates the heat transf ...
The Liquid State
... and gas. Liquids and gases flow while a solid under normal conditions does not. A solid retains its shape while a fluid (a collective name for gases and liquids) will take the shape of the containing vessel. In other words a solid is rigid while fluids do not possess the property of rigidity. The sa ...
... and gas. Liquids and gases flow while a solid under normal conditions does not. A solid retains its shape while a fluid (a collective name for gases and liquids) will take the shape of the containing vessel. In other words a solid is rigid while fluids do not possess the property of rigidity. The sa ...
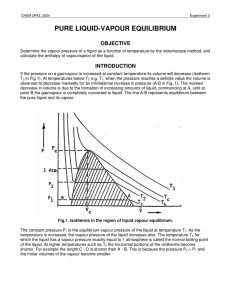
pure liquid-vapour equilibrium - Theoretical and Computational
... The temperature at which the horizontal portion just vanishes is called the CRITICAL TEMPERATURE, Tc, and the corresponding (vapour) pressure is called the CRITICAL PRESSURE, Pc. The shaded area in Fig.1 includes all states of the system which correspond to the existence of two phases, liquid and va ...
... The temperature at which the horizontal portion just vanishes is called the CRITICAL TEMPERATURE, Tc, and the corresponding (vapour) pressure is called the CRITICAL PRESSURE, Pc. The shaded area in Fig.1 includes all states of the system which correspond to the existence of two phases, liquid and va ...
Expt. 5: Binary Phase Diagram CHEM 366 V-1 Binary Solid
... may behave more or less independent of each other but merely diluted, i.e., an ideal solution or mixture, or there may be substantial chemical interaction or complex formation between the constituents. The study of such mixtures can lead to an understanding of the most fundamental intermolecular int ...
... may behave more or less independent of each other but merely diluted, i.e., an ideal solution or mixture, or there may be substantial chemical interaction or complex formation between the constituents. The study of such mixtures can lead to an understanding of the most fundamental intermolecular int ...
EXPLODING BOSE-EINSTEIN CONDENSATES AND - if
... antiparticle terms, which become interesting only at very high temperatures, will not be considered in this approach, because we are focusing our attention in a model of a canonical neutron star, usually said to be a “cold” degenerate neutron gas. One can show that for fixed N , µ is a decreasing fu ...
... antiparticle terms, which become interesting only at very high temperatures, will not be considered in this approach, because we are focusing our attention in a model of a canonical neutron star, usually said to be a “cold” degenerate neutron gas. One can show that for fixed N , µ is a decreasing fu ...
Abstract-Sumer PEKER - ic-rmm1
... flow rate was adjusted with the pressure of the nitrogen gas in the storage tank. The outlet to inlet ratio of the diameters of the converging pipe was, Do/Di = 9 / 21, and the calculated velocity ratio for a HerschelBulkley model fluid would be uo/ui = 5.44. Elongational to shear E/s viscosity ra ...
... flow rate was adjusted with the pressure of the nitrogen gas in the storage tank. The outlet to inlet ratio of the diameters of the converging pipe was, Do/Di = 9 / 21, and the calculated velocity ratio for a HerschelBulkley model fluid would be uo/ui = 5.44. Elongational to shear E/s viscosity ra ...
Packed Bed Reactors - EngineeringDuniya.com
... – Because shear levels are much lower than in STRs ...
... – Because shear levels are much lower than in STRs ...
MACROSCOPIC QUANTUM PHENOMENA FROM PAIRING IN SUPERCONDUCTORS
... Superconductors are remarkable in that they exhibit quantum effects on a broad range of scales. The persistence of current flow in a loop of wire many meters in diameter illustrates that the pairing condensation makes the superfluid wavefunction coherent over macroscopic distances. On the other hand ...
... Superconductors are remarkable in that they exhibit quantum effects on a broad range of scales. The persistence of current flow in a loop of wire many meters in diameter illustrates that the pairing condensation makes the superfluid wavefunction coherent over macroscopic distances. On the other hand ...
Change of state - Mrs. Coyle`s College Chemistry
... Molar heats of fusion are generally much smaller than molar heats of vaporization (liquid molecules are packed closer together and more energy need to rearrange from a solid to liquid) ...
... Molar heats of fusion are generally much smaller than molar heats of vaporization (liquid molecules are packed closer together and more energy need to rearrange from a solid to liquid) ...
Print Activity - Let`s Talk Science
... Is it a liquid or a solid? they do in a normal solid. This makes the goop feel and act as a solid. When the pressure is released the molecules form the random arrangement of a liquid and the goop suddenly flows. Why does it matter? All fluids have a measurable viscosity. Viscosity is the thickness ...
... Is it a liquid or a solid? they do in a normal solid. This makes the goop feel and act as a solid. When the pressure is released the molecules form the random arrangement of a liquid and the goop suddenly flows. Why does it matter? All fluids have a measurable viscosity. Viscosity is the thickness ...
HELIUM - IDC
... Helium is the chemical element with symbol “He” and atomic number 2. It is the second lightest element and the second most abundant element in the universe, representing 23% to 24% of the observable matter (almost all matter that is not hydrogen). Most of the helium is in the form of 4helium isotope ...
... Helium is the chemical element with symbol “He” and atomic number 2. It is the second lightest element and the second most abundant element in the universe, representing 23% to 24% of the observable matter (almost all matter that is not hydrogen). Most of the helium is in the form of 4helium isotope ...
Theoretische Physik IV: Statistische Mechanik, Exercise 6
... (b) Using the result from (a) show that absolute zero cannot be reached by an adiabatic expansion. In the following we gain intuition whether absolute zero can be reached at all. We consider the fact that cooling processes always take place between two curves with X = const., e.g. X1 = P1 , X2 = P2 ...
... (b) Using the result from (a) show that absolute zero cannot be reached by an adiabatic expansion. In the following we gain intuition whether absolute zero can be reached at all. We consider the fact that cooling processes always take place between two curves with X = const., e.g. X1 = P1 , X2 = P2 ...
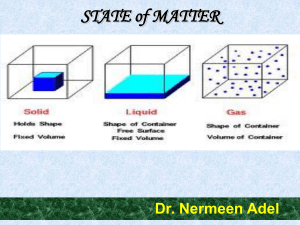
STATE of MATTER
... molecules) are PACKED CLOSELY together. The forces between them are STRONG enough so that they cannot move freely but can only vibrate. • A solid has a stable, definite shape, and a definite volume. • Solids can be changed into liquids by melting, and liquids can be transformed into solids by freezi ...
... molecules) are PACKED CLOSELY together. The forces between them are STRONG enough so that they cannot move freely but can only vibrate. • A solid has a stable, definite shape, and a definite volume. • Solids can be changed into liquids by melting, and liquids can be transformed into solids by freezi ...
Physics 880.06: Problem Set 6
... to an applied uniform ac current density, Jac (t) = Jac x̂ cos(ωt). The second is a frictional force Ff = −ηv, where v is the velocity. The last is a “pinning” force Fpin = −kr per unit vortex length, where r = (x, y) is the two dimensional vector describing the position of the vortex in the xy plan ...
... to an applied uniform ac current density, Jac (t) = Jac x̂ cos(ωt). The second is a frictional force Ff = −ηv, where v is the velocity. The last is a “pinning” force Fpin = −kr per unit vortex length, where r = (x, y) is the two dimensional vector describing the position of the vortex in the xy plan ...
Physics 880.06: Problem Set 6
... to an applied uniform ac current density, Jac (t) = Jac x̂ cos(ωt). The second is a frictional force Ff = −ηv, where v is the velocity. The last is a “pinning” force Fpin = −kr per unit vortex length, where r = (x, y) is the two dimensional vector describing the position of the vortex in the xy plan ...
... to an applied uniform ac current density, Jac (t) = Jac x̂ cos(ωt). The second is a frictional force Ff = −ηv, where v is the velocity. The last is a “pinning” force Fpin = −kr per unit vortex length, where r = (x, y) is the two dimensional vector describing the position of the vortex in the xy plan ...
Lecture 35 (Slides) November 7
... • 1. A total of 3.00 g of liquid water was placed in an evacuated (initially) 12.0L container maintained at a temperature of 80.0 0C. Will a liquid/gas equilibrium be established? What piece of data is required to solve this problem? If no liquid/gas equilibrium is established, determine how much ad ...
... • 1. A total of 3.00 g of liquid water was placed in an evacuated (initially) 12.0L container maintained at a temperature of 80.0 0C. Will a liquid/gas equilibrium be established? What piece of data is required to solve this problem? If no liquid/gas equilibrium is established, determine how much ad ...
HO #15 Maxwell Distribution
... We want to derive a formula for the distribution of velocities of molecules in an ideal gas. We make the following assumptions about the distribution. Let the velocity vector be given by v = (v1 , v2 , v3 ) . Let f1 (v1 ) be the distribution of the component of the velocity in the x-direction, let f ...
... We want to derive a formula for the distribution of velocities of molecules in an ideal gas. We make the following assumptions about the distribution. Let the velocity vector be given by v = (v1 , v2 , v3 ) . Let f1 (v1 ) be the distribution of the component of the velocity in the x-direction, let f ...
Dimples due to dislocations at the superfluid/solid interface of
... 4He crystals may, contain facets (atomically smooth faces) in their equilibrium shape. For a perfect facet, the growth coefficient K is exactly zero: It can grow only owing to fluetuational mechanisms which enable the nucleation of new atomic layers. Since thermal nucleation of layers, dominant at h ...
... 4He crystals may, contain facets (atomically smooth faces) in their equilibrium shape. For a perfect facet, the growth coefficient K is exactly zero: It can grow only owing to fluetuational mechanisms which enable the nucleation of new atomic layers. Since thermal nucleation of layers, dominant at h ...
Chapter 12 - Midway ISD
... What would happen to system if volume of container was suddenly increased? Amount of vapor would now be in lower concentration and thus less condensation would be occurring and more evaporation occurring until system reaches equilibrium again. At the new equilibrium, there fewer liquid molecul ...
... What would happen to system if volume of container was suddenly increased? Amount of vapor would now be in lower concentration and thus less condensation would be occurring and more evaporation occurring until system reaches equilibrium again. At the new equilibrium, there fewer liquid molecul ...
Entropy geometric construction of a pure substance with normal
... ∂T v n ∂p T When any of these conditions is not met, the system suffers a phase transition to enforce stability. Phase transitions are classified into first and second order. In a first order phase transition the extensive quantities s, e, and v are discontinuous while the intensive ones T , p and µ ...
... ∂T v n ∂p T When any of these conditions is not met, the system suffers a phase transition to enforce stability. Phase transitions are classified into first and second order. In a first order phase transition the extensive quantities s, e, and v are discontinuous while the intensive ones T , p and µ ...
vortices - University of Toronto Physics
... by Feynman in the early 1950’s, as fully quantum-mechanical objects. We now know that most of the flow properties of He superfluid are governed by the vortices in them, which can form very complex patterns. They can form closed ‘vortex rings’, which are also quantum objects, and which can tunnel and ...
... by Feynman in the early 1950’s, as fully quantum-mechanical objects. We now know that most of the flow properties of He superfluid are governed by the vortices in them, which can form very complex patterns. They can form closed ‘vortex rings’, which are also quantum objects, and which can tunnel and ...
ln2_storage_pre
... On the other hand, the gas cylinders are probably at room temperature. This is way above the critical temperature for both fluids, so you will not get a liquid no matter how much pressure you put on it. The gases in the cylinders are supercritical fluids, though when you get that far above the criti ...
... On the other hand, the gas cylinders are probably at room temperature. This is way above the critical temperature for both fluids, so you will not get a liquid no matter how much pressure you put on it. The gases in the cylinders are supercritical fluids, though when you get that far above the criti ...
Superfluid helium-4
A superfluid is a state of matter in which the matter behaves like a fluid with zero viscosity and zero entropy. The substance, which looks like a normal liquid, will flow without friction past any surface, which allows it to continue to circulate over obstructions and through pores in containers which hold it, subject only to its own inertia.Known as a major facet in the study of quantum hydrodynamics and macroscopic quantum phenomena, the superfluidity effect was discovered by Pyotr Kapitsa and John F. Allen, and Don Misener in 1937. It has since been described through phenomenological and microscopic theories. The formation of the superfluid is known to be related to the formation of a Bose–Einstein condensate. This is made obvious by the fact that superfluidity occurs in liquid helium-4 at far higher temperatures than it does in helium-3. Each atom of helium-4 is a boson particle, by virtue of its zero spin. Helium-3, however, is a fermion particle, which can form bosons only by pairing with itself at much lower temperatures, in a process similar to the electron pairing in superconductivity.In the 1950s, Hall and Vinen performed experiments establishing the existence of quantized vortex lines in superfluid helium. In the 1960s, Rayfield and Reif established the existence of quantized vortex rings. Packard has observed the intersection of vortex lines with the free surface of the fluid, and Avenel and Varoquaux have studied the Josephson effect in superfluid helium-4. In 2006 a group at the University of Maryland visualized quantized vortices by using small tracer particles of solid hydrogen.