s-BLOCK ELEMENTS - einstein classes
... but higher than that of the alkali metals. There is no regular trend in melting points as we move from top to bottom in the group. They have smaller size than alkali metals and have higher melting point than that of alkali metals. Because they aremore closely packed due to increasing nuclear charge. ...
... but higher than that of the alkali metals. There is no regular trend in melting points as we move from top to bottom in the group. They have smaller size than alkali metals and have higher melting point than that of alkali metals. Because they aremore closely packed due to increasing nuclear charge. ...
Module-2-s-and-d-elements - Львівський національний медичний
... Henry Cavendish synthesized water by detonating a mixture of hydrogen and the air. However, the results of his experiments were not clearly interpreted until two years later, when the French chemist Antoine Laurent Lavoisier proved that water was not an element but a compound of oxygen and hydrogen. ...
... Henry Cavendish synthesized water by detonating a mixture of hydrogen and the air. However, the results of his experiments were not clearly interpreted until two years later, when the French chemist Antoine Laurent Lavoisier proved that water was not an element but a compound of oxygen and hydrogen. ...
KISS Notes
... armed with iron weapons will usually beat a bronzearmed man. Iron tools and even the humble nail allowed new developments in buildings, ships, wagons... remember that towns, trade and commerce give wealth and power. An iron plough allows more land to be cultivated to grow more food, to feed a bigger ...
... armed with iron weapons will usually beat a bronzearmed man. Iron tools and even the humble nail allowed new developments in buildings, ships, wagons... remember that towns, trade and commerce give wealth and power. An iron plough allows more land to be cultivated to grow more food, to feed a bigger ...
The Alkaline Earth Metals (Group 2) - Chemwiki
... 1. To describe how to isolate the alkaline earth metals. 2. To be familiar with the reactions, compounds, and complexes of the alkaline earth metals. Like the alkali metals, the alkaline earth metals are so reactive that they are never found in elemental form in nature. Because they form +2 ions tha ...
... 1. To describe how to isolate the alkaline earth metals. 2. To be familiar with the reactions, compounds, and complexes of the alkaline earth metals. Like the alkali metals, the alkaline earth metals are so reactive that they are never found in elemental form in nature. Because they form +2 ions tha ...
Chapter 23 Metals and Metallurgy
... • Many important metals are included in this group. • The transition metals are the elements in the d block of the periodic table. ...
... • Many important metals are included in this group. • The transition metals are the elements in the d block of the periodic table. ...
Chapter -13 Principles of Metallurgy
... absorb the moisture, if any from the air. Hence the nails are not rusted. From the above experiment, we can prove that air and water are essential for corrosion. ...
... absorb the moisture, if any from the air. Hence the nails are not rusted. From the above experiment, we can prove that air and water are essential for corrosion. ...
Answers - Dr Terry Dwyer National Curriculum mathematics and
... 1 Vertical columns are called Groups. 2 Horizontal rows are called Periods. 3 Elements in the same group, column, tend to have similar properties. If Argon, Ar, is used to help reduce unwanted reactions in welding, what is the atomic number of another element that probably could be used for the s ...
... 1 Vertical columns are called Groups. 2 Horizontal rows are called Periods. 3 Elements in the same group, column, tend to have similar properties. If Argon, Ar, is used to help reduce unwanted reactions in welding, what is the atomic number of another element that probably could be used for the s ...
Group 1: The Alkali Metals
... Alkali metals are the chemical elements found in Group 1 of the periodic table. The alkali metals include: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (RB), Cesium (Cs), and Francium (Fr). Hydrogen, while it appears to be listed within Group 1, is not included in the alkali metals since it ra ...
... Alkali metals are the chemical elements found in Group 1 of the periodic table. The alkali metals include: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (RB), Cesium (Cs), and Francium (Fr). Hydrogen, while it appears to be listed within Group 1, is not included in the alkali metals since it ra ...
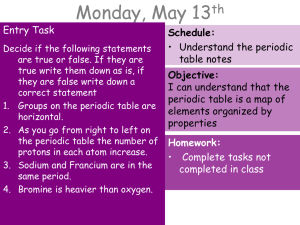
Entry Task
... • Elements that conduct electricity and heat well and have a shiny appearance • Shaped easily by pounding, bending or being drawn into a long wire • Solid at room temperature-except for mercury which is a liquid ...
... • Elements that conduct electricity and heat well and have a shiny appearance • Shaped easily by pounding, bending or being drawn into a long wire • Solid at room temperature-except for mercury which is a liquid ...
Document
... of electrons are available for bonding. There is only a ____________________ in successive ionization energies of the d electrons. Thus when elements of the first transition series react to form compounds, they can form ions of roughly the ___________________ by losing different numbers of electrons ...
... of electrons are available for bonding. There is only a ____________________ in successive ionization energies of the d electrons. Thus when elements of the first transition series react to form compounds, they can form ions of roughly the ___________________ by losing different numbers of electrons ...
Chapter 23 Metals and Metallurgy
... • Many important metals are included in this group. • Comprised of elements in d block of periodic table. ...
... • Many important metals are included in this group. • Comprised of elements in d block of periodic table. ...
the Main-Group Metals - McQuarrie General Chemistry
... electrical properties, magnesium is also used in various electronic devices, such as laptop computers. Like the alkali metals, the alkaline-earth metals show an increasing tendency to form peroxides with increasing size. Strontium peroxide, SrO2(s), is formed at high oxygen pressure; and barium pero ...
... electrical properties, magnesium is also used in various electronic devices, such as laptop computers. Like the alkali metals, the alkaline-earth metals show an increasing tendency to form peroxides with increasing size. Strontium peroxide, SrO2(s), is formed at high oxygen pressure; and barium pero ...
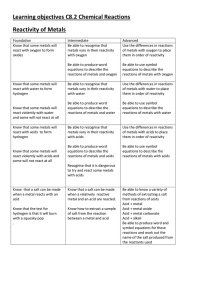
Learning objectives C8.2 Chemical Reactions Reactivity of Metals
... are considered to be air pollutants: Carbon Monoxide, Particulates Nitrogen Oxides, Sulphur dioxide ...
... are considered to be air pollutants: Carbon Monoxide, Particulates Nitrogen Oxides, Sulphur dioxide ...
Preview Sample 1
... 13. The average mass of an atom is determined by A. taking a weighted average of all isotopic masses B. averaging the masses of each isotope C. taking a weighted average of all stable isotopic masses D. adding the isotopic masses and dividing by the number of isotopes ...
... 13. The average mass of an atom is determined by A. taking a weighted average of all isotopic masses B. averaging the masses of each isotope C. taking a weighted average of all stable isotopic masses D. adding the isotopic masses and dividing by the number of isotopes ...
Intermediate 1 Unit 2 Homework 5
... When a metal reacts with oxygen, a metal oxide is produced. E.g. zinc + oxygen ...
... When a metal reacts with oxygen, a metal oxide is produced. E.g. zinc + oxygen ...
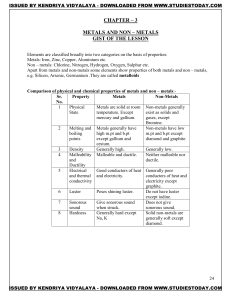
METALS AND NON – METALS Concepts
... Metals: Iron, Zinc, Copper, Aluminium etc. Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc. Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Arsenic, Germanium .They are called metalloids Comparison of physical and chemical ...
... Metals: Iron, Zinc, Copper, Aluminium etc. Non – metals: Chlorine, Nitrogen, Hydrogen, Oxygen, Sulphur etc. Apart from metals and non-metals some elements show properties of both metals and non – metals, e.g. Silicon, Arsenic, Germanium .They are called metalloids Comparison of physical and chemical ...
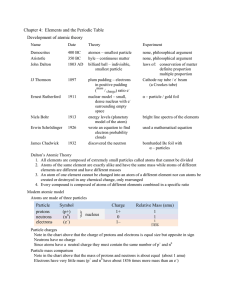
Chapter 4: Elements and the Periodic Table Development of atomic
... Mass number: the number of protons and neutrons in the nucleus of an atom Mass number defines what isotope an atom is Hyphen notation: a means of writing a single nuclide in an isotope Example: carbon–13 has 6 p+ (it is carbon) and 7 n0 (it is one of the nuclides of carbon) Calculating p+, n0, and e ...
... Mass number: the number of protons and neutrons in the nucleus of an atom Mass number defines what isotope an atom is Hyphen notation: a means of writing a single nuclide in an isotope Example: carbon–13 has 6 p+ (it is carbon) and 7 n0 (it is one of the nuclides of carbon) Calculating p+, n0, and e ...
Wet Corrosion Conditions for Wet Corrosion Just as we live in an
... depends on the molecular weight of the active chemical specie. Electrochemical attack, the scientific name for wet corrosion, depends on three circumstances occurring simultaneously. This might seem restrictive, but the evidence of wet corrosion that we see all around us proves that these conditions ...
... depends on the molecular weight of the active chemical specie. Electrochemical attack, the scientific name for wet corrosion, depends on three circumstances occurring simultaneously. This might seem restrictive, but the evidence of wet corrosion that we see all around us proves that these conditions ...
periodic table
... A. similar characteristic properties: bp/fp/sp heat B. same # electrons in the valence shell C. same oxidation #: charge after octet rule applied 4. Zig-zag line between B-Al and Po-At separates metals (80% chart) on the left from non-metals on the right 5. The most reactive: A. metals: bottom left ...
... A. similar characteristic properties: bp/fp/sp heat B. same # electrons in the valence shell C. same oxidation #: charge after octet rule applied 4. Zig-zag line between B-Al and Po-At separates metals (80% chart) on the left from non-metals on the right 5. The most reactive: A. metals: bottom left ...
Objective 3 Stations Student Sheet
... 1. How is the periodic table organized? 2. What family of elements has valence electrons at two energy levels? 3. What are the elements called that are between metals and nonmetals? 4. Which family of nonmetals has seven valence electrons? 5. What are some properties of noble gases? 6. What is anoth ...
... 1. How is the periodic table organized? 2. What family of elements has valence electrons at two energy levels? 3. What are the elements called that are between metals and nonmetals? 4. Which family of nonmetals has seven valence electrons? 5. What are some properties of noble gases? 6. What is anoth ...
Element Symbol
... mixed and cannot be visibly distinguished. The particles of the substances are so small that they cannot be easily seen. 11. Another name for a homogeneous mixture is a solution. ...
... mixed and cannot be visibly distinguished. The particles of the substances are so small that they cannot be easily seen. 11. Another name for a homogeneous mixture is a solution. ...
and View
... c. Symbol—abbreviation of the name. (Greek, Latin, or Location) d. Atomic mass—mass of protons with neutrons. C. IVPAC—organization that chooses permanent name for element. ...
... c. Symbol—abbreviation of the name. (Greek, Latin, or Location) d. Atomic mass—mass of protons with neutrons. C. IVPAC—organization that chooses permanent name for element. ...
ALKALI EARTH METALS Introduction Properties Beryllium
... Alkaline earth metals, after the alkali metals, are secondary metals with strong metallic properties. The group 2A elements are less active than those of 1A, but more active than those of group 3A. Except Be, all form ionic compounds. Be forms mostly covalent compounds. The atomic radius increases f ...
... Alkaline earth metals, after the alkali metals, are secondary metals with strong metallic properties. The group 2A elements are less active than those of 1A, but more active than those of group 3A. Except Be, all form ionic compounds. Be forms mostly covalent compounds. The atomic radius increases f ...