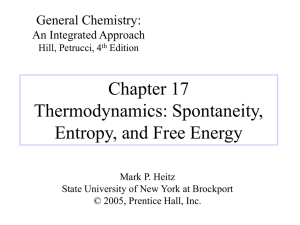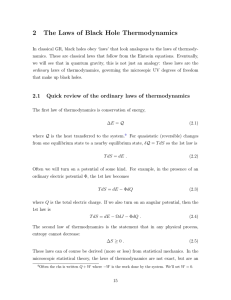
2 The Laws of Black Hole Thermodynamics
... (b) Assume Q > 0. For one sign of q, the energy ✏ can be negative. Which sign? If we drop a negative-energy particle into a black hole, the mass of the black hole decreases. Therefore it is possible to extract energy using this process. For uncharged but rotating black holes, a similar procedure can ...
... (b) Assume Q > 0. For one sign of q, the energy ✏ can be negative. Which sign? If we drop a negative-energy particle into a black hole, the mass of the black hole decreases. Therefore it is possible to extract energy using this process. For uncharged but rotating black holes, a similar procedure can ...
Introduction: basic ideas, equation of state and the first law of
... Physical systems are composed of particles that interact with each other. In everyday life (namely, not in the relativistic or the quantum limits) the motion of the particles and the interactions between them are well described by Newton’s laws. In principle, this approach should be sufficient to de ...
... Physical systems are composed of particles that interact with each other. In everyday life (namely, not in the relativistic or the quantum limits) the motion of the particles and the interactions between them are well described by Newton’s laws. In principle, this approach should be sufficient to de ...
Introduction to Statistical Thermodynamics - cryocourse 2011
... 3) History of Statistical Thermodynamics (http://en.wikipedia.org/wiki/Statistical_thermodynamics) In 1738, Swiss physicist and mathematician Daniel Bernoulli published Hydrodynamica which laid the basis for the kinetic theory of gases. In this work, Bernoulli positioned the argument, still used to ...
... 3) History of Statistical Thermodynamics (http://en.wikipedia.org/wiki/Statistical_thermodynamics) In 1738, Swiss physicist and mathematician Daniel Bernoulli published Hydrodynamica which laid the basis for the kinetic theory of gases. In this work, Bernoulli positioned the argument, still used to ...
Shannon entropy as a measure of uncertainty in positions and
... 2. From the property 0 ≤ Pi ≤ 1, we know that H (P ) ≥ 0. These two properties are essential for the information-like interpretation of the Shannon entropy because information can be neither negative nor expressed in any physical units. Unfortunately, the continuous Shannon entropy does not possess ...
... 2. From the property 0 ≤ Pi ≤ 1, we know that H (P ) ≥ 0. These two properties are essential for the information-like interpretation of the Shannon entropy because information can be neither negative nor expressed in any physical units. Unfortunately, the continuous Shannon entropy does not possess ...
Particle-based Collision Detection
... approximately equal to the number of vertices between particles placed on the model. Experiments show that about 10% of particles, in average, are placed near equilibrium position. By this means Nmin should be about 10 times smaller then number of particles n ...
... approximately equal to the number of vertices between particles placed on the model. Experiments show that about 10% of particles, in average, are placed near equilibrium position. By this means Nmin should be about 10 times smaller then number of particles n ...
Identification - KHAZAR UNIVERSITY
... those having legitimate reasons for absence (illness, family bereavement, etc.) are required to inform the instructor. Tardiness / other disruptions. If a student is late to the class for more than 10 (ten) minutes, (s)he is not allowed to enter and disturb the class. However, this student is able t ...
... those having legitimate reasons for absence (illness, family bereavement, etc.) are required to inform the instructor. Tardiness / other disruptions. If a student is late to the class for more than 10 (ten) minutes, (s)he is not allowed to enter and disturb the class. However, this student is able t ...
Chemistry Review Fill in the blank
... 5. Cations are _________________ charged ions. They are formed by __________________ electrons. 6. Anions are _________________ charged ions. They are formed by __________________ electrons. 7. The periodic table is arranged by ______________________________________. 8. The periodic table is mostly ...
... 5. Cations are _________________ charged ions. They are formed by __________________ electrons. 6. Anions are _________________ charged ions. They are formed by __________________ electrons. 7. The periodic table is arranged by ______________________________________. 8. The periodic table is mostly ...
Document
... To cause a molecule to dissociate into its atoms, we must supply the molecule with enough energy to induce such vigorous vibrations that its atoms fly apart, an endothermic process (∆H > 0). To assess the entropy change, we first note that a molecule has a greater number of available energy levels t ...
... To cause a molecule to dissociate into its atoms, we must supply the molecule with enough energy to induce such vigorous vibrations that its atoms fly apart, an endothermic process (∆H > 0). To assess the entropy change, we first note that a molecule has a greater number of available energy levels t ...