11.3 GAS VOLUMES AND THE IDEAL GAS LAW
... In 1811, Amedeo Avogadro explained Gay-Lussac’s law of combining volumes of gases without violating Dalton’s idea of indivisible atoms. Avogadro reasoned that, instead of always being in monatomic form when they combine to form products, gas molecules can contain more than one atom. He also stated a ...
... In 1811, Amedeo Avogadro explained Gay-Lussac’s law of combining volumes of gases without violating Dalton’s idea of indivisible atoms. Avogadro reasoned that, instead of always being in monatomic form when they combine to form products, gas molecules can contain more than one atom. He also stated a ...
Black Hole Event
... Higher n shows lower multiplicity Hawking temperature is higher One particle carries larger energy ...
... Higher n shows lower multiplicity Hawking temperature is higher One particle carries larger energy ...
lect1f
... reactor (or exiting from the reactor) if the amounts of substances expressed in the reaction equation react at constant temperature, and both the reactants and the products are pure substances at po pressure. ...
... reactor (or exiting from the reactor) if the amounts of substances expressed in the reaction equation react at constant temperature, and both the reactants and the products are pure substances at po pressure. ...
Department of Science - Chemistry
... Explain the relationship between statistical mechanics, the ideal gas equation and the kinetic theory of gases State the assumptions behind collision theory, and outline its strengths and weaknesses State the assumptions behind transition state theory, and interpret the results of transition state t ...
... Explain the relationship between statistical mechanics, the ideal gas equation and the kinetic theory of gases State the assumptions behind collision theory, and outline its strengths and weaknesses State the assumptions behind transition state theory, and interpret the results of transition state t ...
Colloidal Crystal: emergence of long range order from colloidal fluid
... with the colloidal particles so that van der Waals interaction is diminished. Crystallization was carried out by gravitational sedimentation after randomizing the sample by extensive tumbling. Sedimentation occurred slowly and undisturbed, analogous to crystallization of a molecular crystal. The res ...
... with the colloidal particles so that van der Waals interaction is diminished. Crystallization was carried out by gravitational sedimentation after randomizing the sample by extensive tumbling. Sedimentation occurred slowly and undisturbed, analogous to crystallization of a molecular crystal. The res ...
2003 The McGraw-Hill Companies, Inc. All rights reserved. 14
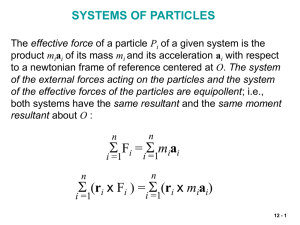
... • Although f ij and f ji are equal and opposite, the work of these forces will not, in general, cancel out. • If the forces acting on the particles are conservative, the work is equal to the change in potential energy and T1 V1 T2 V2 which expresses the principle of conservation of energy for ...
... • Although f ij and f ji are equal and opposite, the work of these forces will not, in general, cancel out. • If the forces acting on the particles are conservative, the work is equal to the change in potential energy and T1 V1 T2 V2 which expresses the principle of conservation of energy for ...
Chapter 6 - DePaul University Department of Chemistry
... • The gas laws are mathematical equations that describe the behavior of gases as they are mixed, subjected to pressure or temperature changes, or allowed to diffuse. • The pressure exerted on or by a gas sample and the temperature of the sample are important quantities in gas law calculations. ...
... • The gas laws are mathematical equations that describe the behavior of gases as they are mixed, subjected to pressure or temperature changes, or allowed to diffuse. • The pressure exerted on or by a gas sample and the temperature of the sample are important quantities in gas law calculations. ...
Exercise in Physical Chemistry
... First Law of Thermodynamics / Internal Energy (YouTube) https://www.youtube.com/watch?v=Xb05CaG7TsQ The Second Law of Thermodynamics (YouTube) https://www.youtube.com/watch?v=4nPGS8xgzKw The Third Law of Thermodynamics (YouTube) https://www.youtube.com/watch?v=hBqcGWzHQbY Using Free Energy (YouYube) ...
... First Law of Thermodynamics / Internal Energy (YouTube) https://www.youtube.com/watch?v=Xb05CaG7TsQ The Second Law of Thermodynamics (YouTube) https://www.youtube.com/watch?v=4nPGS8xgzKw The Third Law of Thermodynamics (YouTube) https://www.youtube.com/watch?v=hBqcGWzHQbY Using Free Energy (YouYube) ...
Physical chemistry 1
... • The reaction system in equilibrium, thermodynamic equilibrium constant. • The dependence of the equilibrium on pressure and temperature, biological activity, thermodynamics of the aerobic and anaerobic metabolism. • The properties of electrolyte solution, the average activity coefficients of elect ...
... • The reaction system in equilibrium, thermodynamic equilibrium constant. • The dependence of the equilibrium on pressure and temperature, biological activity, thermodynamics of the aerobic and anaerobic metabolism. • The properties of electrolyte solution, the average activity coefficients of elect ...
Atomic arrangement, short and long range order, point. Direction
... distances is repeated for atoms separated byany distance—that is, both longrange and short-range order exist. The basic criteria of longrangeorder are the symmetry and regularity of arrangement of particles, which repeat at any distancefrom a given atom. The presence of long-range and shortrange ord ...
... distances is repeated for atoms separated byany distance—that is, both longrange and short-range order exist. The basic criteria of longrangeorder are the symmetry and regularity of arrangement of particles, which repeat at any distancefrom a given atom. The presence of long-range and shortrange ord ...