Experiment Six - Seattle Central College
... might the differences in mass affect the heat capacity? You can compare the values of c and cp for the three metals to answer this question. You should choose one metal, and repeat each measurement three times, allowing the metal to heat in the water bath for at least 30 seconds between trials. (Be ...
... might the differences in mass affect the heat capacity? You can compare the values of c and cp for the three metals to answer this question. You should choose one metal, and repeat each measurement three times, allowing the metal to heat in the water bath for at least 30 seconds between trials. (Be ...
Calorimetry Lab
... C. What was the effect of changing the initial temperature of the copper? ___________ ___________________________________________________________________ ___________________________________________________________________ 4. Draw conclusions: The amount that the water’s temperature increases depends ...
... C. What was the effect of changing the initial temperature of the copper? ___________ ___________________________________________________________________ ___________________________________________________________________ 4. Draw conclusions: The amount that the water’s temperature increases depends ...
Ch_15
... You heat a half-cup of tea and its temperature rises by 4C. How much will the temperature rise if you add the same amount of heat to a full cup of tea? ...
... You heat a half-cup of tea and its temperature rises by 4C. How much will the temperature rise if you add the same amount of heat to a full cup of tea? ...
How Water and Energy Usage is Measured
... Benchmarking: Determining Water and Energy-Use Efficiency Benchmarking is an important component of any energy management program because it helps you establish a baseline of energy or water use. ...
... Benchmarking: Determining Water and Energy-Use Efficiency Benchmarking is an important component of any energy management program because it helps you establish a baseline of energy or water use. ...
The efficiency of a microwave oven
... Radar uses microwaves because it can pass through cloud and dust and is reflected back by the metal surfaces of planes. ...
... Radar uses microwaves because it can pass through cloud and dust and is reflected back by the metal surfaces of planes. ...
When it gets colder – heating cables to protect against cold
... flow of heat is caused by electrical energy which is converted into heat in the heating conductor. The heat then flows out through the insulation into the surrounding medium. ...
... flow of heat is caused by electrical energy which is converted into heat in the heating conductor. The heat then flows out through the insulation into the surrounding medium. ...
How to calculate the Heat / Molar Heat of Combustion
... b. Calculate the total rise in temperature of the water. Show your work below. ...
... b. Calculate the total rise in temperature of the water. Show your work below. ...
Q = mcAT - nnhsrasetti
... If a substance receives heat and experiences an increase in temperature then Q is a positive number and ΔT is a positive number. If a substance loses heat and experiences a decrease in temperature then Q is a negative number and ΔT is a negative number. Q (heat energy) can be measured in eithe ...
... If a substance receives heat and experiences an increase in temperature then Q is a positive number and ΔT is a positive number. If a substance loses heat and experiences a decrease in temperature then Q is a negative number and ΔT is a negative number. Q (heat energy) can be measured in eithe ...
Modelling of wire die coating
... transfer coefficient between a free streaming water film and metal corrugated surface. The relation between relevant parameters which determine the heat transfer coefficient has been obtained by means of Wilson-plot method using data from the measurement. ...
... transfer coefficient between a free streaming water film and metal corrugated surface. The relation between relevant parameters which determine the heat transfer coefficient has been obtained by means of Wilson-plot method using data from the measurement. ...
specific heat
... The method of mixtures makes use of the principles that when two bodies at different temperatures exchange heat, the quantity of heat lost by the warmer body is equal to the heat gained by the cooler body, and some intermediate equilibrium temperature is finally reached. This is true provided no hea ...
... The method of mixtures makes use of the principles that when two bodies at different temperatures exchange heat, the quantity of heat lost by the warmer body is equal to the heat gained by the cooler body, and some intermediate equilibrium temperature is finally reached. This is true provided no hea ...
test and comparison of hot water stores for solar combisystems
... flaps, is placed above the solar loop heat exchanger. In order to provide the high temperatures, which are required for building up a good stratification, this store has to be connected to a system where the collector loop works according to the lowflow principle. The water returning from the space ...
... flaps, is placed above the solar loop heat exchanger. In order to provide the high temperatures, which are required for building up a good stratification, this store has to be connected to a system where the collector loop works according to the lowflow principle. The water returning from the space ...
Intro to Physics Lab
... temperature range between room temperature (water) and water boiling temperature (metal). To compare the accepted values of specific heat for 2 different metals ...
... temperature range between room temperature (water) and water boiling temperature (metal). To compare the accepted values of specific heat for 2 different metals ...
Dr. McCord Calorimetry
... be very abstract. In a solution with water as the solvent, I can define the system as only those species that are reactants and products – all of which are dissolved in water (the surroundings). This is what is so nice about a coffee-cup calorimeter. It is incredibly simple and cheap. Pull a cup off ...
... be very abstract. In a solution with water as the solvent, I can define the system as only those species that are reactants and products – all of which are dissolved in water (the surroundings). This is what is so nice about a coffee-cup calorimeter. It is incredibly simple and cheap. Pull a cup off ...
Specific Heat and Phase Change - CK
... • The amount of heat capacitance (and thus its specific heat value) is related to something called ’degrees of freedom,’ which basically says how free is the object to move in different ways (and thus how much kinetic energy can it store inside itself without breaking apart). For example, solids hav ...
... • The amount of heat capacitance (and thus its specific heat value) is related to something called ’degrees of freedom,’ which basically says how free is the object to move in different ways (and thus how much kinetic energy can it store inside itself without breaking apart). For example, solids hav ...
Enthalpy of combustion
... 3. 0.1g of methanol, CH3OH, was burned and the heat given out used to raise the temperature of 100 cm3 of water at 21oC. Use the enthalpy of combustion of methanol in the data booklet to calculate the final temperature of the water. 4. 0.2g of methane, CH4, was burned and the heat given out used to ...
... 3. 0.1g of methanol, CH3OH, was burned and the heat given out used to raise the temperature of 100 cm3 of water at 21oC. Use the enthalpy of combustion of methanol in the data booklet to calculate the final temperature of the water. 4. 0.2g of methane, CH4, was burned and the heat given out used to ...
Solutions Student Handout
... chemical reaction from 30˚C to 50˚C. Specific heat capacity of water is 4.18J/˚C g. q= mc∆T = 100g (4.18J/˚C g) (50˚C− 30˚C) = 8360 J Ex. 2 Water and chemical 80g of sodium hydroxide dissolves in 120g of water and causes an increase in temperature from 20˚C to 30˚C. Calculate the heat of the reactio ...
... chemical reaction from 30˚C to 50˚C. Specific heat capacity of water is 4.18J/˚C g. q= mc∆T = 100g (4.18J/˚C g) (50˚C− 30˚C) = 8360 J Ex. 2 Water and chemical 80g of sodium hydroxide dissolves in 120g of water and causes an increase in temperature from 20˚C to 30˚C. Calculate the heat of the reactio ...
Chapter 2 Boiler 101: typical NYC residential heating system
... On the other hand, hot water systems are slower to deliver heat than steam systems as they have a smaller reservoir of heat. They can have higher electricity consumption because of the power required by the circulating pumps to keep the water flowing in the water loop. Hot water systems have general ...
... On the other hand, hot water systems are slower to deliver heat than steam systems as they have a smaller reservoir of heat. They can have higher electricity consumption because of the power required by the circulating pumps to keep the water flowing in the water loop. Hot water systems have general ...
Return to Lab Menu
... inside the cell through with the regulation of various enzymes is nothing more than the combustion of glucose. So the energy released gradually inside the cell is overall the same energy that would be released if you simply burned glucose. In the cell it is just released in small manageable incremen ...
... inside the cell through with the regulation of various enzymes is nothing more than the combustion of glucose. So the energy released gradually inside the cell is overall the same energy that would be released if you simply burned glucose. In the cell it is just released in small manageable incremen ...
Experiment 6 ~ Joule Heating of a Resistor
... When a resistor absorbs electrical energy, it dissipates this energy in the form of heat Q. If the resistor is placed in the calorimeter, the amount of heat produced can be measured when it is absorbed in the calorimeter. Consider the experimental arrangement shown in Figure 5.1, which a resistor co ...
... When a resistor absorbs electrical energy, it dissipates this energy in the form of heat Q. If the resistor is placed in the calorimeter, the amount of heat produced can be measured when it is absorbed in the calorimeter. Consider the experimental arrangement shown in Figure 5.1, which a resistor co ...
appendix d - Florida Building Commission
... 2.1 Scope. This standard applies to desuperheater/water heaters supplied as separate components, as defined in Section 3.1 for residential potable water heating. 2.2 Exclusion. This standard does not apply to desupereater/water heaters supplied as components of factory assembled refrigeration or air ...
... 2.1 Scope. This standard applies to desuperheater/water heaters supplied as separate components, as defined in Section 3.1 for residential potable water heating. 2.2 Exclusion. This standard does not apply to desupereater/water heaters supplied as components of factory assembled refrigeration or air ...
Introduction - HCC Learning Web
... Specific Heat is the amount of heat required to raise the temperature of 1 gram of substance by one degree Celsius. It can be expressed in terms of calories/(gm∙C) or joules/ (kg∙K) . Water has a relatively high specific heat of 1cal/(gm∙C). Metals usually have a low specific heat, for example lea ...
... Specific Heat is the amount of heat required to raise the temperature of 1 gram of substance by one degree Celsius. It can be expressed in terms of calories/(gm∙C) or joules/ (kg∙K) . Water has a relatively high specific heat of 1cal/(gm∙C). Metals usually have a low specific heat, for example lea ...
6 Physical Properties and Principles I. Review of Fundamental
... The SI unit of pressure is the pascal, symbol Pa, the special name given to a pressure of one newton per square metre (N/m2). The relationships between the pascal and some other pressure units are shown in the table but note that not all are, or can be, expressed exactly. Note also that the term st ...
... The SI unit of pressure is the pascal, symbol Pa, the special name given to a pressure of one newton per square metre (N/m2). The relationships between the pascal and some other pressure units are shown in the table but note that not all are, or can be, expressed exactly. Note also that the term st ...
Lab 9: Specific Heat ( )T
... 14.5 °C to 15.5°C. This is because careful measurements of the energy transferred revealed that the specific heat of water was not constant for all temperature ranges. This is also true for the metals we have investigated in this experiment! Come up with an experiment (or modify this experiment) in ...
... 14.5 °C to 15.5°C. This is because careful measurements of the energy transferred revealed that the specific heat of water was not constant for all temperature ranges. This is also true for the metals we have investigated in this experiment! Come up with an experiment (or modify this experiment) in ...
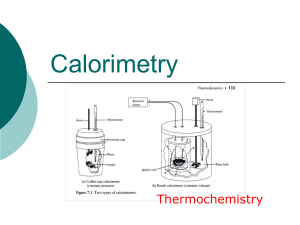
Thermochemistry - hrsbstaff.ednet.ns.ca
... water, thermometer… Heavily insulated or vacuum insulated so no convection or conduction can occur affecting the enthalpy of the system. Often use the equation: q=CΔT ...
... water, thermometer… Heavily insulated or vacuum insulated so no convection or conduction can occur affecting the enthalpy of the system. Often use the equation: q=CΔT ...
ICEST2015 Paper Template
... rather than to its conversion to electrical energy. The primary forms of direct use include heating and cooling. Geothermal energy could be used to supply hot water or could be used with a special equipment (radiators) to make buildings warmer during winter seasons. In general, the geothermal fluid ...
... rather than to its conversion to electrical energy. The primary forms of direct use include heating and cooling. Geothermal energy could be used to supply hot water or could be used with a special equipment (radiators) to make buildings warmer during winter seasons. In general, the geothermal fluid ...
Water heating
Water heating is a thermodynamic process that uses an energy source to heat water above its initial temperature. Typical domestic uses of hot water include cooking, cleaning, bathing, and space heating. In industry, hot water and water heated to steam have many uses.Domestically, water is traditionally heated in vessels known as water heaters, kettles, cauldrons, pots, or coppers. These metal vessels that heat a batch of water do not produce a continual supply of heated water at a preset temperature. Rarely, hot water occurs naturally, usually from natural hot springs. The temperature varies based on the consumption rate, becoming cooler as flow increases.Appliances that provide a continual supply of hot water are called water heaters, hot water heaters, hot water tanks, boilers, heat exchangers, geysers, or calorifiers. These names depend on region, and whether they heat potable or non-potable water, are in domestic or industrial use, and their energy source. In domestic installations, potable water heated for uses other than space heating is also called domestic hot water (DHW).Fossil fuels (natural gas, liquefied petroleum gas, oil), or solid fuels are commonly used for heating water. These may be consumed directly or may produce electricity that, in turn, heats water. Electricity to heat water may also come from any other electrical source, such as nuclear power or renewable energy. Alternative energy such as solar energy, heat pumps, hot water heat recycling, and geothermal heating can also heat water, often in combination with backup systems powered by fossil fuels or electricity.Densely populated urban areas of some countries provide district heating of hot water. This is especially the case in Scandinavia and Finland. District heating systems supply energy for water heating and space heating from waste heat from industries, power plants, incinerators, geothermal heating, and central solar heating. Actual heating of tap water is performed in heat exchangers at the consumers' premises. Generally the consumer has no in-building backup system, due to the expected high availability of district heating systems.