2 - TestBankTop
... observing how a charged drop of oil falls in the presence and in the absence of an electric field. An atomizer introduces a fine mist of oil drops into the top chamber (Figure 2.6). Several drops happen to fall through a small hole into the lower chamber, where the experimenter follows the motion of ...
... observing how a charged drop of oil falls in the presence and in the absence of an electric field. An atomizer introduces a fine mist of oil drops into the top chamber (Figure 2.6). Several drops happen to fall through a small hole into the lower chamber, where the experimenter follows the motion of ...
Atoms, Molecules, and Ions
... observing how a charged drop of oil falls in the presence and in the absence of an electric field. An atomizer introduces a fine mist of oil drops into the top chamber (Figure 2.6). Several drops happen to fall through a small hole into the lower chamber, where the experimenter follows the motion of ...
... observing how a charged drop of oil falls in the presence and in the absence of an electric field. An atomizer introduces a fine mist of oil drops into the top chamber (Figure 2.6). Several drops happen to fall through a small hole into the lower chamber, where the experimenter follows the motion of ...
Worksheet to accompany demos on exchange reactions
... tell if a reaction were an oxidation-reduction reaction by looking to see if any reactants end up more positive or more negative after chemical change (i.e., compare a (monatomic) reactant to its corresponding product). I will revisit and clarify this point again below when discussing chemical speci ...
... tell if a reaction were an oxidation-reduction reaction by looking to see if any reactants end up more positive or more negative after chemical change (i.e., compare a (monatomic) reactant to its corresponding product). I will revisit and clarify this point again below when discussing chemical speci ...
Lecture 5 - Course Notes
... fluctuations when there is no magnetic field. • The moments align parallel to the field when magnetic field is applied. • Susceptibility is positive but very small for paramagnetic materials ...
... fluctuations when there is no magnetic field. • The moments align parallel to the field when magnetic field is applied. • Susceptibility is positive but very small for paramagnetic materials ...
Basics of Material Sciences - E
... Subshells have same energy but different _______ (1) Size (2) Shape (3) Orientation (4) All ...
... Subshells have same energy but different _______ (1) Size (2) Shape (3) Orientation (4) All ...
Semiconductor
... covalent bond structure and therefore a hole in the valence band of the energy level diagram. Every impurity atom will produce a hole in the valence band. These holes will drift to produce an electrical current if a voltage is applied to the material and the P type semiconductor is a much better con ...
... covalent bond structure and therefore a hole in the valence band of the energy level diagram. Every impurity atom will produce a hole in the valence band. These holes will drift to produce an electrical current if a voltage is applied to the material and the P type semiconductor is a much better con ...
Total view of the AFM
... maintained at ~10-8 Torr vacuum. • Microscopes are usually operated in the voltage range of 20 – 30 keV, but for insulating samples 1 kV or less can be used. For insulating samples a thin metal coating can also be used. • The standard electron detector is an EverhartThornley design that is capable o ...
... maintained at ~10-8 Torr vacuum. • Microscopes are usually operated in the voltage range of 20 – 30 keV, but for insulating samples 1 kV or less can be used. For insulating samples a thin metal coating can also be used. • The standard electron detector is an EverhartThornley design that is capable o ...
Write this into your supplemental packet opposite page
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
... 5. Predict the transition metal cation charge for iron, Fe, in the ionic salt Fe 2 (SO4 )3 , and place it in the cation box below. 6. Give a name for Fe 2 (SO4 )3 . Since transition metals can variable charge, you must some how indicate metal cation charge in its name. ...
Powder X-Ray Diffraction
... X-rays are electromagnetic radiation of wavelength about 1 Å (10-10 m), which is about the same size as an atom. They occur in that portion of the electromagnetic spectrum between gamma-rays and the ultraviolet. The discovery of X-rays in 1895 enabled scientists to probe crystalline structure at the ...
... X-rays are electromagnetic radiation of wavelength about 1 Å (10-10 m), which is about the same size as an atom. They occur in that portion of the electromagnetic spectrum between gamma-rays and the ultraviolet. The discovery of X-rays in 1895 enabled scientists to probe crystalline structure at the ...
University of Groningen Designing molecular nano
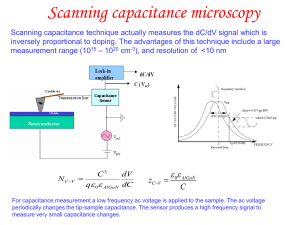
... acquiring dI/dV spectra on a grid of points that raster the surface with the advantage of having the complete energy spectra (in a selected range) available at each location. In this modality, the feedback-loop is deactivated during acquisition and can be periodically reactivated when the tip moves ...
... acquiring dI/dV spectra on a grid of points that raster the surface with the advantage of having the complete energy spectra (in a selected range) available at each location. In this modality, the feedback-loop is deactivated during acquisition and can be periodically reactivated when the tip moves ...
Cleaning Up With Atom Economy
... Green Chemistry Principle: Atom Economy Atom economy means maximizing the incorporation of material from the starting materials or reagents into the final product. It is essentially pollution prevention at the molecular level. For example, a chemist practicing atom economy would choose to synthesize ...
... Green Chemistry Principle: Atom Economy Atom economy means maximizing the incorporation of material from the starting materials or reagents into the final product. It is essentially pollution prevention at the molecular level. For example, a chemist practicing atom economy would choose to synthesize ...
2 - TEST BANK 360
... observing how a charged drop of oil falls in the presence and in the absence of an electric field. An atomizer introduces a fine mist of oil drops into the top chamber (Figure 2.6). Several drops happen to fall through a small hole into the lower chamber, where the experimenter follows the motion of ...
... observing how a charged drop of oil falls in the presence and in the absence of an electric field. An atomizer introduces a fine mist of oil drops into the top chamber (Figure 2.6). Several drops happen to fall through a small hole into the lower chamber, where the experimenter follows the motion of ...
2.ATOMS, MOLECULES, AND IONS
... electron by observing how a charged drop of oil falls in the presence and in the absence of an electric field. An atomizer introduces a fine mist of oil drops into the top chamber (Figure 2.6). Several drops happen to fall through a small hole into the lower chamber where the experimenter follows th ...
... electron by observing how a charged drop of oil falls in the presence and in the absence of an electric field. An atomizer introduces a fine mist of oil drops into the top chamber (Figure 2.6). Several drops happen to fall through a small hole into the lower chamber where the experimenter follows th ...
uplift luna ap chemistry
... phosphate is PO43−, then phosphite must be PO33−. You can also use the prefixes hypo- and perwith the chlorate series. Perchlorate, ClO4−, was really “hyper and -ate yet another oxygen” when compared to chlorate, ClO3−. Hypochlorite is a double whammy. It is -ite and therefore “ate” one less oxygen ...
... phosphate is PO43−, then phosphite must be PO33−. You can also use the prefixes hypo- and perwith the chlorate series. Perchlorate, ClO4−, was really “hyper and -ate yet another oxygen” when compared to chlorate, ClO3−. Hypochlorite is a double whammy. It is -ite and therefore “ate” one less oxygen ...
ANALYSIS OF THE SILVER GROUP CATIONS
... isolated from the solution containing the other metal cations, the three insoluble chlorides can be separated from one another by chemical means. To do this, we exploit differences in the chemistry of the three ions according to the separation scheme given on a separate sheet and in the table. As yo ...
... isolated from the solution containing the other metal cations, the three insoluble chlorides can be separated from one another by chemical means. To do this, we exploit differences in the chemistry of the three ions according to the separation scheme given on a separate sheet and in the table. As yo ...
Chapter 4 Nomenclature and Chemical Equations
... Depending on the type of bonds present in a compound, different rules are applied to its naming. ...
... Depending on the type of bonds present in a compound, different rules are applied to its naming. ...
(111) direction : molecular field parameters
... temperature only some of the states of the rare-earth are populated. The garnet in question is magnetically saturated in moderate fields at temperatures near 0 K and as such it belongs to the category in which the rare earth moment is locked in by crystalline field effects. Such a phenomenon has bee ...
... temperature only some of the states of the rare-earth are populated. The garnet in question is magnetically saturated in moderate fields at temperatures near 0 K and as such it belongs to the category in which the rare earth moment is locked in by crystalline field effects. Such a phenomenon has bee ...
View - Workshops+SJCOE Workshop Management
... organize this information. The substructure of atoms determines how they combine and rearrange to form all of the world’s substances. Electrical attractions and repulsions between charged particles (i.e., atomic nuclei and electrons) in matter explain the structure of atoms and the forces between at ...
... organize this information. The substructure of atoms determines how they combine and rearrange to form all of the world’s substances. Electrical attractions and repulsions between charged particles (i.e., atomic nuclei and electrons) in matter explain the structure of atoms and the forces between at ...
C6 - NuPECC
... relevant for the average power. For example, at 5 MW proton beam power the time average cold neutron flux of coupled liquid H2 moderators achieves the steady flux of the most powerful reactor in existence. Its instantaneous "peak" flux during the pulses, which matters mainly for most experiments, ca ...
... relevant for the average power. For example, at 5 MW proton beam power the time average cold neutron flux of coupled liquid H2 moderators achieves the steady flux of the most powerful reactor in existence. Its instantaneous "peak" flux during the pulses, which matters mainly for most experiments, ca ...
Physical Setting/Chemistry Examination
... The answers to the questions in Part B–2 and Part C are to be written in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your ...
... The answers to the questions in Part B–2 and Part C are to be written in your separate answer booklet. Be sure to fill in the heading on the front of your answer booklet. Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your ...
The Low Energy Ion Assisted Control of Interfacial Structure: Ion Incident Angle Effects
... permalloy) layers [1±3]. The eect can be observed as a large (5±50%) change in resistance to the propagation of a small electric current through the layers when a magnetic ®eld is applied. It occurs because of a change in spin dependent electron scattering when the relative orientation of the magne ...
... permalloy) layers [1±3]. The eect can be observed as a large (5±50%) change in resistance to the propagation of a small electric current through the layers when a magnetic ®eld is applied. It occurs because of a change in spin dependent electron scattering when the relative orientation of the magne ...
Nanostructure calculation of CoAg core
... a monolayer Ag. By increasing the initial cobalt concentration to values above 70%, the Ag shell starts to be perforated along the edges of the Co core. For clusters with a diameter of about 2.8 nm, the mi nimum silver concentration to cover the whole Co surface is thus given by about 30%. In order ...
... a monolayer Ag. By increasing the initial cobalt concentration to values above 70%, the Ag shell starts to be perforated along the edges of the Co core. For clusters with a diameter of about 2.8 nm, the mi nimum silver concentration to cover the whole Co surface is thus given by about 30%. In order ...
Experimental and Theoretical Charge Density Analysis of a
... electron distribution, a precise charge density analysis (either experimental or theoretical) is a method of choice to recover molecular properties. In particular, it is of interest to know how the two CH2 carbon units present in compound BEST between the bromine and the sulfur atom are different fro ...
... electron distribution, a precise charge density analysis (either experimental or theoretical) is a method of choice to recover molecular properties. In particular, it is of interest to know how the two CH2 carbon units present in compound BEST between the bromine and the sulfur atom are different fro ...
Frequency Dependence of Polarization: When a dielectric is placed
... 3.29 and demonstrates hysteresis. This behavior is similar to that produced by a ferromagnet when it is cycled through an alternating magnetic field. The description is based on the domain structure of ferroelectrics. When the dipoles in a crystal are randomly oriented there is no net P. When a fiel ...
... 3.29 and demonstrates hysteresis. This behavior is similar to that produced by a ferromagnet when it is cycled through an alternating magnetic field. The description is based on the domain structure of ferroelectrics. When the dipoles in a crystal are randomly oriented there is no net P. When a fiel ...
Atom probe

The atom probe was introduced at the 14th International Field Emission Symposium in 1967 by Erwin W. Müller and John Panitz. For the first time an instrument could “... determine the nature of one single atom seen on a metal surface and selected from neighboring atoms at the discretion of the observer”. Erwin Wilhelm Müller, J. A. Panitz, and S. Brooks McLane. The atom probe is closely related to the field ion microscope, the first microscopic instrument capable of atomic resolution, developed in 1951 by Erwin Wilhelm Müller.Atom probes are unlike conventional optical or electron microscopes, in that the magnification effect comes from the magnification provided by a highly curved electric field, rather than by the manipulation of radiation paths. The method is destructive in nature removing ions from a sample surface in order to image and identify them, generating magnifications sufficient to observe individual atoms as they are removed from the sample surface. Through coupling of this magnification method with time of flight mass spectrometry, ions evaporated by application of electric pulses can have their mass-to-charge ratio computed.Through successive evaporation of material, layers of atoms are removed from a specimen, allowing for probing not only of the surface, but also through the material itself. Computer methods are utilised to rebuild a three-dimensional view of the sample, prior to it being evaporated, providing atomic scale information on the structure of a sample, as well as providing the type atomic species information. The instrument allows the three-dimensional reconstruction of up to billions of atoms from a sharp tip (corresponding to specimen volumes of 10,000-10,000,000 nm3).