EFFECTS OF BIOREACTOR OPERATION PARAMETERS ON
... organic acids with the constant air inlet and agitation rate .................... 62 4.5.a The variations in oxygen transfer parameters with cultivation time at LimOT, LOT1 and LOT2 conditions ...................................................... 66 4.5.b The variations in oxygen transfer parameter ...
... organic acids with the constant air inlet and agitation rate .................... 62 4.5.a The variations in oxygen transfer parameters with cultivation time at LimOT, LOT1 and LOT2 conditions ...................................................... 66 4.5.b The variations in oxygen transfer parameter ...
dhaA - Queen`s University Belfast
... Figure 3: 2D-PAGE gels of regions selected from the ‘other fraction’ – not containing the NarAa and Ab components – but containing putative reductase and ferredoxin components - from a MonoQ column. A– pyruvate –grown cells without induction of naphthalene associated genes; B- naphthalene- grown cel ...
... Figure 3: 2D-PAGE gels of regions selected from the ‘other fraction’ – not containing the NarAa and Ab components – but containing putative reductase and ferredoxin components - from a MonoQ column. A– pyruvate –grown cells without induction of naphthalene associated genes; B- naphthalene- grown cel ...
Chapter 9 Review, pages 628–633
... (c) In a molecule of HCN(g), the carbon and nitrogen atoms share 3 pairs of electrons. The nitrogen atom is more electronegative, so it has a greater attraction for the electrons than the carbon atom. We can assume that the nitrogen atom has taken the 3 electrons from the carbon atom. Thus the oxid ...
... (c) In a molecule of HCN(g), the carbon and nitrogen atoms share 3 pairs of electrons. The nitrogen atom is more electronegative, so it has a greater attraction for the electrons than the carbon atom. We can assume that the nitrogen atom has taken the 3 electrons from the carbon atom. Thus the oxid ...
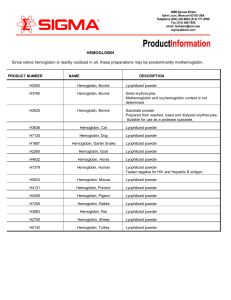
HEMOGLOBIN Since native hemoglobin is readily oxidized in air
... erythrocytes have been published. 1 PRODUCT DESCRIPTION: Hemoglobin is the major component of red blood cells, and is responsible for their red color. Its normal concentration in erythrocytes is 34%. Hemoglobin is the most important respiratory protein of vertebrates by virtue of its ability to tran ...
... erythrocytes have been published. 1 PRODUCT DESCRIPTION: Hemoglobin is the major component of red blood cells, and is responsible for their red color. Its normal concentration in erythrocytes is 34%. Hemoglobin is the most important respiratory protein of vertebrates by virtue of its ability to tran ...
Formatted - RESPIRATION
... atmosphere and CO2 concentration is just 0.033%. Molecules of a gas move from its higher concentration to lower concentration. In plants, during daytime, the leaf cells take CO2 and release O2 in photosynthesis. At the same time in respiration, O2 is utilized and CO2 is given out. However, since pho ...
... atmosphere and CO2 concentration is just 0.033%. Molecules of a gas move from its higher concentration to lower concentration. In plants, during daytime, the leaf cells take CO2 and release O2 in photosynthesis. At the same time in respiration, O2 is utilized and CO2 is given out. However, since pho ...
Positional cues for the starch/lipid balance in maize kernels and
... partitioning of sucrose to non-starch storage compounds. Starch storage in endosperm, however, appears specifically adapted to the localized low-oxygen environment inside these, and possibly other grains. Results Oxygen is severely depleted inside developing maize kernels Experiments were performed ...
... partitioning of sucrose to non-starch storage compounds. Starch storage in endosperm, however, appears specifically adapted to the localized low-oxygen environment inside these, and possibly other grains. Results Oxygen is severely depleted inside developing maize kernels Experiments were performed ...
Nitrogen and Oxygen Family
... with itself and with other elements having small size and high electronegativity (e.g., C, O). Heavier elements of this group do not form p–p bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping. Thus, nitrogen exists as a diatomic molecule with a tri ...
... with itself and with other elements having small size and high electronegativity (e.g., C, O). Heavier elements of this group do not form p–p bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping. Thus, nitrogen exists as a diatomic molecule with a tri ...
Kinetics of growth and sugar consumption in yeasts 63: 343-352, 1993.
... yeasts' as hosts for heterologous gene expression, including broader substrate specificity, availability of strong inducible promoters, absence of aerobic alcoholic fermentation (i.e. absence of the Crabtree effect), etc. However, a major factor, decisive for the use of alternative yeasts in commerc ...
... yeasts' as hosts for heterologous gene expression, including broader substrate specificity, availability of strong inducible promoters, absence of aerobic alcoholic fermentation (i.e. absence of the Crabtree effect), etc. However, a major factor, decisive for the use of alternative yeasts in commerc ...
Effect of Potassium on Sol-Gel Cerium and Lanthanum Oxide
... of diesel fuel that remains in the filters of vehicle engines, causing serious health problems [9]. An alternative is to burn this particulate material [7] [9]. The catalyst deposited in the ceramic filters could oxidize the soot, reducing its emission into the atmosphere. However, the temperatures ...
... of diesel fuel that remains in the filters of vehicle engines, causing serious health problems [9]. An alternative is to burn this particulate material [7] [9]. The catalyst deposited in the ceramic filters could oxidize the soot, reducing its emission into the atmosphere. However, the temperatures ...
Extended Abstract
... characterization of many substances, beginning with the oxyacids. Lavoisier’s theory of the gaseous state led to explanations for heat and light produced from combustion processes and rang the death knell for the phlogiston theory. Other workers, notably Joseph Black, had discovered “fixed air” (car ...
... characterization of many substances, beginning with the oxyacids. Lavoisier’s theory of the gaseous state led to explanations for heat and light produced from combustion processes and rang the death knell for the phlogiston theory. Other workers, notably Joseph Black, had discovered “fixed air” (car ...
Blood Agents
... 300,000 tons of cyanide is produced annually. It is used in printing, agriculture, photography, and in the manufacture of paper and plastics. It is also a combustion product of burning synthetic materials. Rail cars with 30,000-gallon tanks of cyanide represent potential transportation and terrorist ...
... 300,000 tons of cyanide is produced annually. It is used in printing, agriculture, photography, and in the manufacture of paper and plastics. It is also a combustion product of burning synthetic materials. Rail cars with 30,000-gallon tanks of cyanide represent potential transportation and terrorist ...
Effects of nitrous oxide on diazepam sedation of young children
... returned to normal whenthe stimulus ended. In regard to respiratory rate, similar transitory changes (for example, from 24 to 40) occurred at a time of particular stimulus. Adverseeffects Two subjects coughed up small amounts of fluid during treatment and one spit up slightly at the end of treatment ...
... returned to normal whenthe stimulus ended. In regard to respiratory rate, similar transitory changes (for example, from 24 to 40) occurred at a time of particular stimulus. Adverseeffects Two subjects coughed up small amounts of fluid during treatment and one spit up slightly at the end of treatment ...
Role of the non-respiratory pathways in the utilization of molecular
... (AOX), located on the matrix side of the inner mitochondrial membrane and encoded by a member of the AOX gene family.179 The constitutive or inducible AOX bypasses the cytochrome chain by directly transferring electrons from ubiquinol to oxygen. The alternative respiration is also called ‘ubiquinol ...
... (AOX), located on the matrix side of the inner mitochondrial membrane and encoded by a member of the AOX gene family.179 The constitutive or inducible AOX bypasses the cytochrome chain by directly transferring electrons from ubiquinol to oxygen. The alternative respiration is also called ‘ubiquinol ...
Nicotine Increases Hepatic Oxygen Uptake in the Isolated Perfused
... from endogenous glycogen (Ross et al., 1967). Basal rates of glucose output as well as lactate ⫹ pyruvate production were both around 100 mol/g/h (Table 1). Infusion of nicotine decreased rates of glucose output slightly, whereas rates of glycolysis were decreased dramatically (Fig. 1B). Average ba ...
... from endogenous glycogen (Ross et al., 1967). Basal rates of glucose output as well as lactate ⫹ pyruvate production were both around 100 mol/g/h (Table 1). Infusion of nicotine decreased rates of glucose output slightly, whereas rates of glycolysis were decreased dramatically (Fig. 1B). Average ba ...
Oxidative Phosphorylation accompanying Oxidation of
... the incubation was carried out with a gas phase of air. Oxygen consumption was followed until approximately 8,umoles were taken up, when the reaction was stopped by the addition of 0-3ml. of trichloroacetic acid (10%, w/v). The inorganic phosphate present before and after the addition of hexokinase ...
... the incubation was carried out with a gas phase of air. Oxygen consumption was followed until approximately 8,umoles were taken up, when the reaction was stopped by the addition of 0-3ml. of trichloroacetic acid (10%, w/v). The inorganic phosphate present before and after the addition of hexokinase ...
Honors Chemistry: Ch. 12 – Stoichiometry Some useful terms
... 4.) Calculate the mass of silver needed to react with chlorine to produce 84 g of silver chloride (Hint: Write a balanced equation first). 5.) Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at STP. 2N2(g) + 3O2(g) 2N2O3(g) 6. ...
... 4.) Calculate the mass of silver needed to react with chlorine to produce 84 g of silver chloride (Hint: Write a balanced equation first). 5.) Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at STP. 2N2(g) + 3O2(g) 2N2O3(g) 6. ...
Solutions_C19
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
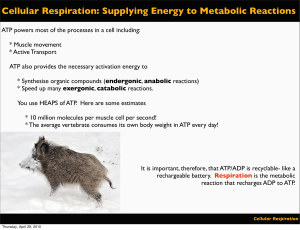
Cellular Respiration: Supplying Energy to Metabolic Reactions
... Coenzymes are relatively small organic non-protein molecules that catalyse reactions by acting as carriers of electrons and protons. They may also carry specific atoms or groups of atoms, such as phosphate, that are required for, or produced by, chemical reactions. The coenzymes we study in VCE Biol ...
... Coenzymes are relatively small organic non-protein molecules that catalyse reactions by acting as carriers of electrons and protons. They may also carry specific atoms or groups of atoms, such as phosphate, that are required for, or produced by, chemical reactions. The coenzymes we study in VCE Biol ...
Cu(II)–disulfide complexes display simultaneous superoxide
... study for MnTBAP was always lower than the values estimated for each of the studied Cu(II)–RSSR complexes. In addition to the kinetic rate constants, Table 1 provides data on the ability of the Cu(II)–RSSR complexes to inhibit by 50% the rate of reduction of Cyt c (I50). The I50 value is the concent ...
... study for MnTBAP was always lower than the values estimated for each of the studied Cu(II)–RSSR complexes. In addition to the kinetic rate constants, Table 1 provides data on the ability of the Cu(II)–RSSR complexes to inhibit by 50% the rate of reduction of Cyt c (I50). The I50 value is the concent ...
Variation in the link between oxygen consumption and ATP
... inner mitochondrial membrane. The dissipation of Dp is associated with an increase in heat production [46]. Several distinct biological mechanisms can alter Dp and hence affect the P/O ratio. The composition of the mitochondrial inner membrane, both in terms of its phospholipid fatty acids and the o ...
... inner mitochondrial membrane. The dissipation of Dp is associated with an increase in heat production [46]. Several distinct biological mechanisms can alter Dp and hence affect the P/O ratio. The composition of the mitochondrial inner membrane, both in terms of its phospholipid fatty acids and the o ...
Solutions_C19
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
... 10. Assign oxidation numbers to hydrogen and nitrogen based on the LDS number for ammonia. 10A. The nitrogen atom shares a pair of electrons with each of the three hydrogen atoms. Nitrogen is the more electronegative element because it is farther to the right on the periodic table than hydrogen. Thi ...
Plant and Soil
... histidine and serine) affect growth and nitrogen fixation differently in Azospirillum spp. Amino acids may influence growth and nitrogen fixation of Azospirillum in the association with plants. Azospirillum brasilense and A. halopraeferens are the more osmotolerant species. They utilize most amino a ...
... histidine and serine) affect growth and nitrogen fixation differently in Azospirillum spp. Amino acids may influence growth and nitrogen fixation of Azospirillum in the association with plants. Azospirillum brasilense and A. halopraeferens are the more osmotolerant species. They utilize most amino a ...
PDF
... In the last few years our interest has been devoted to the energy metabolism of the eggs of the common toad Bufo arenarum Hensel which, like some other amphibian eggs, can cleave at a normal rate in the absence of oxygen or in the presence of cyanide (Barbieri & Legname, 1957). Under anaerobic condi ...
... In the last few years our interest has been devoted to the energy metabolism of the eggs of the common toad Bufo arenarum Hensel which, like some other amphibian eggs, can cleave at a normal rate in the absence of oxygen or in the presence of cyanide (Barbieri & Legname, 1957). Under anaerobic condi ...
ABSTRACT Title of Document: A COMPARISON OF THE PHYSIOLOGY
... lower energetic yield, will produce 1.5 molecules of ATP. The net energy yield for complete oxidation of glucose to CO2 and H2O is approximately 30 ATP. When there are not enough oxygen molecules present to act as terminal electron acceptors in cells, the cells cannot create sufficient energy to mai ...
... lower energetic yield, will produce 1.5 molecules of ATP. The net energy yield for complete oxidation of glucose to CO2 and H2O is approximately 30 ATP. When there are not enough oxygen molecules present to act as terminal electron acceptors in cells, the cells cannot create sufficient energy to mai ...
File - Mrs. Roy`s Science Class
... 2H2 + O2 2H2O Two moles of hydrogen and one mole of oxygen form two moles of water. 2 Al2O3 Al + 3O2 2 moles of aluminum oxide decompose to form 4 moles of aluminum and 3 moles of oxygen. ...
... 2H2 + O2 2H2O Two moles of hydrogen and one mole of oxygen form two moles of water. 2 Al2O3 Al + 3O2 2 moles of aluminum oxide decompose to form 4 moles of aluminum and 3 moles of oxygen. ...
Oxygen
Oxygen is a chemical element with symbol O and atomic number 8. It is a member of the chalcogen group on the periodic table and is a highly reactive nonmetallic element and oxidizing agent that readily forms compounds (notably oxides) with most elements. Photosynthesis releases oxygen, and respiration consumes oxygen. Changes in phosphate are related to changes in oxygen concentrations.Oxygen was discovered independently by Carl Wilhelm Scheele, in Uppsala, in 1773 or earlier, and Joseph Priestley in Wiltshire, in 1774, but Priestley is often given priority because his work was published first. The name oxygen was coined in 1777 by Antoine Lavoisier, whose experiments with oxygen helped to discredit the then-popular phlogiston theory of combustion and corrosion. Its name derives from the Greek roots ὀξύς oxys, ""acid"", literally ""sharp"", referring to the sour taste of acids and -γενής -genes, ""producer"", literally ""begetter"", because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition.