THERMODYNAMICS. Elements of Physical Chemistry. By P. Atkins
... (ie. at equlb. at every stage of the process) PfVf Irreversible reaction V ...
... (ie. at equlb. at every stage of the process) PfVf Irreversible reaction V ...
THERMODYNAMICS. Elements of Physical Chemistry. By P. Atkins
... (ie. at equlb. at every stage of the process) PfVf Irreversible reaction V ...
... (ie. at equlb. at every stage of the process) PfVf Irreversible reaction V ...
Determination of the Molar Volume of a Gas KClO3 breaks down
... Attach the test tube to the set-up as indicated in the drawing and spread the contents over the bottom 2 inches of the tube. Open the clamp. A few drops of water may drain from the tube into the beaker, but the flow should stop quickly. If water continues to drain, there is a leak in the system and ...
... Attach the test tube to the set-up as indicated in the drawing and spread the contents over the bottom 2 inches of the tube. Open the clamp. A few drops of water may drain from the tube into the beaker, but the flow should stop quickly. If water continues to drain, there is a leak in the system and ...
Document
... Heat is defined in thermodynamics as the quantity of energy that flows across the boundary between the system and surroundings because of a temperature difference between the system and the surroundings. •Heat is transitory, in that it only appears during a change in state of the system and surroun ...
... Heat is defined in thermodynamics as the quantity of energy that flows across the boundary between the system and surroundings because of a temperature difference between the system and the surroundings. •Heat is transitory, in that it only appears during a change in state of the system and surroun ...
PowerPoint Template
... 1. Describe three commonplace examples of how work is done on or by a system 2. A plumber of mass 65 kg carries a toolbox of mass 15 kg to a fifth floor walkup apartment 15 m above ground level. Calculate the work required for this ...
... 1. Describe three commonplace examples of how work is done on or by a system 2. A plumber of mass 65 kg carries a toolbox of mass 15 kg to a fifth floor walkup apartment 15 m above ground level. Calculate the work required for this ...
PowerPoint Template
... 1. Describe three commonplace examples of how work is done on or by a system 2. A plumber of mass 65 kg carries a toolbox of mass 15 kg to a fifth floor walkup apartment 15 m above ground level. Calculate the work required for this ...
... 1. Describe three commonplace examples of how work is done on or by a system 2. A plumber of mass 65 kg carries a toolbox of mass 15 kg to a fifth floor walkup apartment 15 m above ground level. Calculate the work required for this ...
Other useful things to know about atoms
... o Carbon atoms are unique in that they form strong carbon-carbon bonds allowing the formation of chains, rings and networks of almost infinite variety. There are many more carbon compounds than all other compounds put together and most of these are the basis of life as we know it. It is a useful exe ...
... o Carbon atoms are unique in that they form strong carbon-carbon bonds allowing the formation of chains, rings and networks of almost infinite variety. There are many more carbon compounds than all other compounds put together and most of these are the basis of life as we know it. It is a useful exe ...
2016 - Specimen Paper 4 - Cambridge International Examinations
... (a) Nitrogen is a gas at room temperature. Nitrogen molecules, N2, are spread far apart and move in a random manner at high speed. (i) Draw the electronic structure of a nitrogen molecule. Show only the outer electron shells. ...
... (a) Nitrogen is a gas at room temperature. Nitrogen molecules, N2, are spread far apart and move in a random manner at high speed. (i) Draw the electronic structure of a nitrogen molecule. Show only the outer electron shells. ...
dx cx dx and x - Cameron University
... 4.3 Given a multiple variable expression, determine the partial derivatives with respect to the variables 4.4 Determine the total differential of a function given sufficient information 5.1 State and apply the van der Waals equation of state 5.2 State the differences at the molecular level between a ...
... 4.3 Given a multiple variable expression, determine the partial derivatives with respect to the variables 4.4 Determine the total differential of a function given sufficient information 5.1 State and apply the van der Waals equation of state 5.2 State the differences at the molecular level between a ...
PROPERTIES OF SOLUTIONS
... Solubility of a gas in a liquid is a function of pressure Solubilities of liquids and solids are not affected by pressure The solubility of a gas is directly proportional to the partial pressure of the gas above the solution Henry’s Law: Cg = k Pg where C is the solubility of the gas , P is ...
... Solubility of a gas in a liquid is a function of pressure Solubilities of liquids and solids are not affected by pressure The solubility of a gas is directly proportional to the partial pressure of the gas above the solution Henry’s Law: Cg = k Pg where C is the solubility of the gas , P is ...
Section 1 and 2
... 1- the unknown is the density () of F2 2- since the gas is at relatively low pressure we assume it behaves ideally (so use equation PV = nRT) ...
... 1- the unknown is the density () of F2 2- since the gas is at relatively low pressure we assume it behaves ideally (so use equation PV = nRT) ...
Ch 8 LAN 7th Intro Chem Gases Liquids and Solids
... 1. What are the major intermolecular forces, and how do they affect the states of matter? Be able to explain dipole–dipole forces, London dispersion forces, and hydrogen bonding, recognize which of these forces affect a given molecule, and how these forces are related to the physical properties of ...
... 1. What are the major intermolecular forces, and how do they affect the states of matter? Be able to explain dipole–dipole forces, London dispersion forces, and hydrogen bonding, recognize which of these forces affect a given molecule, and how these forces are related to the physical properties of ...
Chemistry 3510: Physical Chemistry Midterm Exam 1 19 February 2007 Name:
... at a temperature of 625K is carried through a cycle consisting of the following legs: (a) an isothermal reversible expansion to a final volume of 25L; (b) a reversible adiabatic compression to a volume of 5L at a pressure of 4 bar; and (c) an isochoric reversible heating back to the initial conditio ...
... at a temperature of 625K is carried through a cycle consisting of the following legs: (a) an isothermal reversible expansion to a final volume of 25L; (b) a reversible adiabatic compression to a volume of 5L at a pressure of 4 bar; and (c) an isochoric reversible heating back to the initial conditio ...
Lecture 35 (Slides) November 7
... • The normal boiling point of water is 100 oC. At this temperature the vapor pressure of water is exactly 760mm Hg (the normal average atmospheric pressure at sea level). If we heat water in a sealed container all of the steam that is formed is trapped above the liquid water. The additional steam fo ...
... • The normal boiling point of water is 100 oC. At this temperature the vapor pressure of water is exactly 760mm Hg (the normal average atmospheric pressure at sea level). If we heat water in a sealed container all of the steam that is formed is trapped above the liquid water. The additional steam fo ...
chm3400testfin
... 4. (24 points) The density of a mixture of carbon monoxide (CO, M = 28.01 g/mol) and propane (C 3H8, M = 44.11 g/mol) was measured at T = 29.3 C and p = 1.045 atm, and found to be = 1.324 g/L. a) Based on this above information, and assuming the gases obey the ideal gas law, find XCO, the mole f ...
... 4. (24 points) The density of a mixture of carbon monoxide (CO, M = 28.01 g/mol) and propane (C 3H8, M = 44.11 g/mol) was measured at T = 29.3 C and p = 1.045 atm, and found to be = 1.324 g/L. a) Based on this above information, and assuming the gases obey the ideal gas law, find XCO, the mole f ...
Vapor Pressure of a Pure Liquid
... and enter the gas phase until the pressure of the vapor in the bulb reaches a definite value which is determined by the nature of the liquid and its temperature. This is called the vapor pressure of the liquid. In this experiment, the variation of vapor pressure with temperature will be measured and ...
... and enter the gas phase until the pressure of the vapor in the bulb reaches a definite value which is determined by the nature of the liquid and its temperature. This is called the vapor pressure of the liquid. In this experiment, the variation of vapor pressure with temperature will be measured and ...
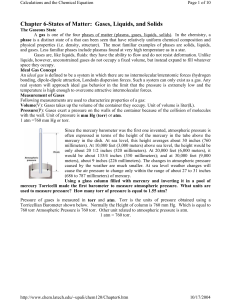
Chapter 6-States of Matter: Gases, Liquids, and Solids
... According to the National Academy of Sciences, the Earth's surface temperature has risen by about 1 degree Fahrenheit in the past century, with accelerated warming during the past two decades. There is new and stronger evidence that most of the Global warming over the last 50 years is attributable t ...
... According to the National Academy of Sciences, the Earth's surface temperature has risen by about 1 degree Fahrenheit in the past century, with accelerated warming during the past two decades. There is new and stronger evidence that most of the Global warming over the last 50 years is attributable t ...
Gases Honors
... – Ex: As ice melts in a closed container, the temperature of the ice-water mixture remains 0C, even though heat energy is being transferred. – Ex: As water boils in a closed container and water vaporizes, the gas-liquid mixture remains 100C, even though heat energy is being transferred. ...
... – Ex: As ice melts in a closed container, the temperature of the ice-water mixture remains 0C, even though heat energy is being transferred. – Ex: As water boils in a closed container and water vaporizes, the gas-liquid mixture remains 100C, even though heat energy is being transferred. ...
Gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture would contain a variety of pure gases much like the air. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer. The interaction of gas particles in the presence of electric and gravitational fields are considered negligible as indicated by the constant velocity vectors in the image. One type of commonly known gas is steam.The gaseous state of matter is found between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super cooled to incredibly low temperatures are classified by their statistical behavior as either a Bose gas or a Fermi gas. For a comprehensive listing of these exotic states of matter see list of states of matter.