Chapter 1 - Gordon State College
... • Mixtures – combinations of 2 or more substances (ex. sugar in water) • 2 Types of Mixtures • 1. Homogenous Mixtures (solutions) = 1 phase • 2. Heterogeneous Mixtures = > 2 phases ...
... • Mixtures – combinations of 2 or more substances (ex. sugar in water) • 2 Types of Mixtures • 1. Homogenous Mixtures (solutions) = 1 phase • 2. Heterogeneous Mixtures = > 2 phases ...
Solutions
... will dissolve at a specific temperature it is saturated. In this case the amount dissolved will be directly on the line of solubility. Solution equilibrium. When a solution contains more than the maximum amount of solute that will dissolve at a specific temperature it is supersaturated. In this case ...
... will dissolve at a specific temperature it is saturated. In this case the amount dissolved will be directly on the line of solubility. Solution equilibrium. When a solution contains more than the maximum amount of solute that will dissolve at a specific temperature it is supersaturated. In this case ...
Exam 4 - Chemistry Courses
... compounds that are believed to be less harmful to the environment. What mass of this substance must evaporate (at constant T) in order to freeze 2 moles of water at 20 °C to ice at 0 °C? The heat of vaporization of CCl2F2 is 289 J/g, the heat of fusion of water is 334 J/g , and the specific heat of ...
... compounds that are believed to be less harmful to the environment. What mass of this substance must evaporate (at constant T) in order to freeze 2 moles of water at 20 °C to ice at 0 °C? The heat of vaporization of CCl2F2 is 289 J/g, the heat of fusion of water is 334 J/g , and the specific heat of ...
Equation of state - Wikipedia, the free encyclopedia
... Although usually not the most convenient equation of state, the virial equation is important because it can be derived directly from statistical mechanics. This equation is also called the Kamerlingh Onnes equation. If appropriate assumptions are made about the mathematical form of intermolecular fo ...
... Although usually not the most convenient equation of state, the virial equation is important because it can be derived directly from statistical mechanics. This equation is also called the Kamerlingh Onnes equation. If appropriate assumptions are made about the mathematical form of intermolecular fo ...
Mole-Mass Conversions
... Unlike mole-particle conversions where the conversion factor is always 1mole = _______________ particles, each mole-mass conversion factor is _________________ to the substance involved. Convert 2.3 moles of sodium (Na) to grams of Na ...
... Unlike mole-particle conversions where the conversion factor is always 1mole = _______________ particles, each mole-mass conversion factor is _________________ to the substance involved. Convert 2.3 moles of sodium (Na) to grams of Na ...
thermodynamic states
... Above integration result implies existence of new function of states, S ...
... Above integration result implies existence of new function of states, S ...
Liquid-gas transition of neon in quasi-one
... is the mechanism invoked to induce a phase gas-liquid27,28 or a melting29,30 transition. There is even an estimation of a gas-liquid transition critical temperature in the case of Ne, using a 3D modified anisotropic Ising model to get T c . 31 A comparison between the number obtained in that approxi ...
... is the mechanism invoked to induce a phase gas-liquid27,28 or a melting29,30 transition. There is even an estimation of a gas-liquid transition critical temperature in the case of Ne, using a 3D modified anisotropic Ising model to get T c . 31 A comparison between the number obtained in that approxi ...
AP Chemistry Chapter 1: Chemical Foundations
... Change in the form of a substance, not in its chemical composition. • A physical change will not break up compounds Example: boiling or freezing water Distillation Filtration Chromatography ...
... Change in the form of a substance, not in its chemical composition. • A physical change will not break up compounds Example: boiling or freezing water Distillation Filtration Chromatography ...
Temperature and solid properties effects on gas–liquid mass transfer
... The knowledge about the effect of solid phase properties, at different temperatures, on gas–liquid transfer and the respective physical mechanisms has been poorly studied. In the present work, the temperature and solid properties (size and density) effects on the gas–liquid mass transfer characteris ...
... The knowledge about the effect of solid phase properties, at different temperatures, on gas–liquid transfer and the respective physical mechanisms has been poorly studied. In the present work, the temperature and solid properties (size and density) effects on the gas–liquid mass transfer characteris ...
FLUID-SOLID SEPARATION_Adsorption
... Physical properties of adsorbent -In the form of small pellets, beads, or granules ranging 0.1 mm to 12 mm in size. -Adsorbent particle has a very porous structure, with many fine pores and pore volumes up to 50% of total particle volume. -Adsorption occurs as a monolayer although several layers so ...
... Physical properties of adsorbent -In the form of small pellets, beads, or granules ranging 0.1 mm to 12 mm in size. -Adsorbent particle has a very porous structure, with many fine pores and pore volumes up to 50% of total particle volume. -Adsorption occurs as a monolayer although several layers so ...
Lecture Notes in Physical Chemistry Semester 2: Kinetics and
... are v x , v y , and v z . Its speed is v = (v x2 + v 2y + v z2 ) 2 , and its translational energy is εtr = 12 mv 2 , where m is the molecule’s mass. The x component of its momentum is mv x . When the molecule collides with a wall parallel to the y z plane, let us assume that the x component of its v ...
... are v x , v y , and v z . Its speed is v = (v x2 + v 2y + v z2 ) 2 , and its translational energy is εtr = 12 mv 2 , where m is the molecule’s mass. The x component of its momentum is mv x . When the molecule collides with a wall parallel to the y z plane, let us assume that the x component of its v ...
co2 removal from natural gas by hydrate formation
... varied in the range of 30.1 to 46.4 bar. In all experiments, the additives concentrations are equal to 4 wt% THF and 3000 ppm SDS, respectively, and the target (or final) temperature is 2.1 °C. The decrease in pressure due to the hydrate formation corresponds to the times greater than 130 min. One c ...
... varied in the range of 30.1 to 46.4 bar. In all experiments, the additives concentrations are equal to 4 wt% THF and 3000 ppm SDS, respectively, and the target (or final) temperature is 2.1 °C. The decrease in pressure due to the hydrate formation corresponds to the times greater than 130 min. One c ...
Sample pages 2 PDF
... namely the sum of potential and kinetic energy, is conserved, i.e., remains constant, only if all acting forces are conservative. If dissipative forces are present, the energy appears not to have been conserved. However, this non-conservation is only apparent due to the fact that other forms of ener ...
... namely the sum of potential and kinetic energy, is conserved, i.e., remains constant, only if all acting forces are conservative. If dissipative forces are present, the energy appears not to have been conserved. However, this non-conservation is only apparent due to the fact that other forms of ener ...
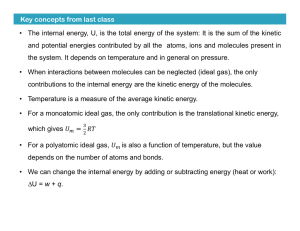
Key concepts from last class • The internal energy, U, is the total
... • For a monoatomic ideal gas, the only contribution is the translational kinetic energy, ...
... • For a monoatomic ideal gas, the only contribution is the translational kinetic energy, ...
Chapter 15
... Patients are usually given intravenous fluids that are isotonic— these solutions have the same osmotic pressure as blood. There are three scenarios: (1) if the external solution is hypertonic, its osmotic pressure > Π(internal), and there is a net flow of water out of the cell. (2) if the external s ...
... Patients are usually given intravenous fluids that are isotonic— these solutions have the same osmotic pressure as blood. There are three scenarios: (1) if the external solution is hypertonic, its osmotic pressure > Π(internal), and there is a net flow of water out of the cell. (2) if the external s ...
Physcal Chemistry ERT 108 semester II 2010/2011
... An adiabatic (isolated) system is one that does not permit the passage of energy as heat through its boundary even if there is a temperature difference between the system and its surroundings. It has adiabatic ...
... An adiabatic (isolated) system is one that does not permit the passage of energy as heat through its boundary even if there is a temperature difference between the system and its surroundings. It has adiabatic ...
Concentration of solutions
... • Vapor pressure lowering. Boiling point is higher and freezing point of a solution is lower than that of a pure solvent. This is due to the presence of nonvolatile solutes. These are substances that have little tendency to become a gas under existing conditions. ...
... • Vapor pressure lowering. Boiling point is higher and freezing point of a solution is lower than that of a pure solvent. This is due to the presence of nonvolatile solutes. These are substances that have little tendency to become a gas under existing conditions. ...
Gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture would contain a variety of pure gases much like the air. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer. The interaction of gas particles in the presence of electric and gravitational fields are considered negligible as indicated by the constant velocity vectors in the image. One type of commonly known gas is steam.The gaseous state of matter is found between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super cooled to incredibly low temperatures are classified by their statistical behavior as either a Bose gas or a Fermi gas. For a comprehensive listing of these exotic states of matter see list of states of matter.