2nd Nine Weeks Notes
... a. The equation shows how the concentration of A depends on time. if the initial concentration of A and the rate constant k are known, the concentration of A at any time can be calculated. ...
... a. The equation shows how the concentration of A depends on time. if the initial concentration of A and the rate constant k are known, the concentration of A at any time can be calculated. ...
Chap 4 - Bakersfield College
... – You will find it important to be able to identify an acid or base as strong or weak. – When you write an ionic equation, strong acids and bases are represented as separate ions. – Weak acids and bases are represented as undissociated “molecules” in ionic equations. ...
... – You will find it important to be able to identify an acid or base as strong or weak. – When you write an ionic equation, strong acids and bases are represented as separate ions. – Weak acids and bases are represented as undissociated “molecules” in ionic equations. ...
Course : Chem 401F
... Chairman of the relevant examination committee within three weeks from the last class held. The examination committee shall send a copy of consolidated marks for each of the viva-voce examination, class assessment, lab. evealuation and practical examinations to the controller of examinations ...
... Chairman of the relevant examination committee within three weeks from the last class held. The examination committee shall send a copy of consolidated marks for each of the viva-voce examination, class assessment, lab. evealuation and practical examinations to the controller of examinations ...
variable specific heat theory
... specific heat depends on temperature changes. Specific heat of real gases also to some extent depends upon the pressure. For real gases the specific heats and hence y are not constant with temperature as in the case of perfect gases (y = 1-67 for monatomic, 7 = 1-4 for diatomic, and y = 1-3 for poly ...
... specific heat depends on temperature changes. Specific heat of real gases also to some extent depends upon the pressure. For real gases the specific heats and hence y are not constant with temperature as in the case of perfect gases (y = 1-67 for monatomic, 7 = 1-4 for diatomic, and y = 1-3 for poly ...
AP Chemistry - Pompton Lakes School District
... Why is dimensional Dimensional analysis is the process through which we convert analysis considered a the measurements we use on a daily basis to the science lab. It process not a fact? can be used with varying units to fit the situation. How do the terms Accuracy refers to how close an exper ...
... Why is dimensional Dimensional analysis is the process through which we convert analysis considered a the measurements we use on a daily basis to the science lab. It process not a fact? can be used with varying units to fit the situation. How do the terms Accuracy refers to how close an exper ...
Research on Hydrogenation of FAME to Fatty Alcohols
... biodiesel industry. However, the choice of target products and technologic routes for producing them should follow three principles: 1) biomass feedstocks are cheaper than petroleum products when they are used as raw materials, 2) process being developed is simple and environmentally-friendly, 3) th ...
... biodiesel industry. However, the choice of target products and technologic routes for producing them should follow three principles: 1) biomass feedstocks are cheaper than petroleum products when they are used as raw materials, 2) process being developed is simple and environmentally-friendly, 3) th ...
Physical Chemistry Lab Report Rubric –Veldman Fall 2012
... the sample to be burned, which will first be benzoic acid then sucrose, in a metal crucible. A small length of wire, about 10 cm, feeds through and is in direct contact with the sample. An electric current is passed through the wire, heating it rapidly, thus initiating combustion. The bomb must then ...
... the sample to be burned, which will first be benzoic acid then sucrose, in a metal crucible. A small length of wire, about 10 cm, feeds through and is in direct contact with the sample. An electric current is passed through the wire, heating it rapidly, thus initiating combustion. The bomb must then ...
Homogeneous Catalysis
... An assortment of case studies is provided and the authors work through each one in great detail, giving an overall perspective on the science. Finally, very useful and informative appendices and a glossary are provided together with a comprehensive index that is good enough to rival any search engin ...
... An assortment of case studies is provided and the authors work through each one in great detail, giving an overall perspective on the science. Finally, very useful and informative appendices and a glossary are provided together with a comprehensive index that is good enough to rival any search engin ...
Chapter 16 Controlling the yield of reactions
... a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0.44 mol of H2 and 0.44 mol of I2 were present. Calculate the value of the equilibrium constant at this temperature. c A third mixture consisted of ...
... a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0.44 mol of H2 and 0.44 mol of I2 were present. Calculate the value of the equilibrium constant at this temperature. c A third mixture consisted of ...
Document
... ΔSuniverse is zero; therefore, the reaction is an equilibrium process at 98°C. In fact, this is the melting point of sodium. ...
... ΔSuniverse is zero; therefore, the reaction is an equilibrium process at 98°C. In fact, this is the melting point of sodium. ...
Direct production of hydrogen peroxide from CO, O2, and H2O over
... Table 1 shows the catalytic results for H2O2 production from CO/O2/H2O over several types of metal nanoparticles dispersed on alumina prepared by the wet reduction (WR) method, which has recently been shown to be an effective method for the preparation of various amorphous alloy catalysts for versati ...
... Table 1 shows the catalytic results for H2O2 production from CO/O2/H2O over several types of metal nanoparticles dispersed on alumina prepared by the wet reduction (WR) method, which has recently been shown to be an effective method for the preparation of various amorphous alloy catalysts for versati ...
Geochemistry 303
... The solution to problem 4 is to use the compositions from the log file on the part of the equilibria you can calculate or from a nearby equilibria you can calculate. If it’s the first line on a diagram, have a guess from another “tcd” file in the same system or use your rock ...
... The solution to problem 4 is to use the compositions from the log file on the part of the equilibria you can calculate or from a nearby equilibria you can calculate. If it’s the first line on a diagram, have a guess from another “tcd” file in the same system or use your rock ...
Competition for Electrons
... track of electrons based on the arbitrary assumption that shared electrons belong to the more electronegative element n Rules for assigning oxidation numbers q Oxidation numbers for atoms that are free elements are always zero q The oxidation numbers of ions are the same as the charge on the ion q S ...
... track of electrons based on the arbitrary assumption that shared electrons belong to the more electronegative element n Rules for assigning oxidation numbers q Oxidation numbers for atoms that are free elements are always zero q The oxidation numbers of ions are the same as the charge on the ion q S ...
Procedures for the disposal of liquid chemical residues and
... Stockholm Vatten’s wastewater treatment plant in Henriksdal through the sewage system. These guidelines constitute an exception to the rule that no hazardous substances may be poured down the drain, and have been approved by the City of Stockholm's Environment and Health Administration and Stockholm ...
... Stockholm Vatten’s wastewater treatment plant in Henriksdal through the sewage system. These guidelines constitute an exception to the rule that no hazardous substances may be poured down the drain, and have been approved by the City of Stockholm's Environment and Health Administration and Stockholm ...
Chapter 1: Aqueous Processing Systems
... For many years the study of the electronic structure and properties of solids has been regarded as the preserve of physicists. Chemists, who spend a good deal of time and effort looking at electronic structures of molecules, have tended to avoid these aspects of solids. The situation is changing, ho ...
... For many years the study of the electronic structure and properties of solids has been regarded as the preserve of physicists. Chemists, who spend a good deal of time and effort looking at electronic structures of molecules, have tended to avoid these aspects of solids. The situation is changing, ho ...
advanced chemistry may 2011 marking scheme
... produce three equal bonds, repulsion between electron density of which is equal thus generating a species with bond angle of 120o. (2 marks) (d) Excess hydrochloric acid was poured on 0.2 g sodium carbonate and the gas was collected at 298 K. Calculate the volume in cm3 if the gas was measured at a ...
... produce three equal bonds, repulsion between electron density of which is equal thus generating a species with bond angle of 120o. (2 marks) (d) Excess hydrochloric acid was poured on 0.2 g sodium carbonate and the gas was collected at 298 K. Calculate the volume in cm3 if the gas was measured at a ...
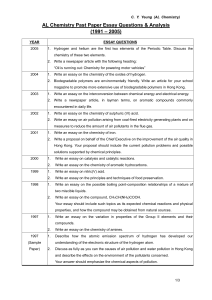
AL Chemistry Past paper essay questions
... Write an essay on amino acids, polypeptides and proteins. Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
... Write an essay on amino acids, polypeptides and proteins. Your essay should include the properties of amino acids in aqueous solutions and a method of separation for a mixture of amino acids, as well as the constitution of polypeptides and proteins and their hydrolysis. ...
Chemical Sensors and Biosensors. Analytical Techniques in the Sciences (AnTs) * Brochure
... an up–to–the–minute overview of a wide range of sensing systems, discussing the elements of different transducers used in sensors and the selective elements that are employed. The style is relatively non–mathematical and informal in approach. Key features and subjects covered include the following: ...
... an up–to–the–minute overview of a wide range of sensing systems, discussing the elements of different transducers used in sensors and the selective elements that are employed. The style is relatively non–mathematical and informal in approach. Key features and subjects covered include the following: ...
Chapter 1: Matter and Measurement
... Heats of Reaction and Calorimetry Work The First Law of Thermodynamics Heats of Reaction: U and H The Indirect Determination of H: Hess’s Law Standard Enthalpies of Formation Fuels as Sources of Energy Focus On Fats, Carbohydrates and Energy Storage ...
... Heats of Reaction and Calorimetry Work The First Law of Thermodynamics Heats of Reaction: U and H The Indirect Determination of H: Hess’s Law Standard Enthalpies of Formation Fuels as Sources of Energy Focus On Fats, Carbohydrates and Energy Storage ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.