TEKS Presentation Properties of Matter
... Characteristics of a substance that are observed when it reacts (changes) to produce one or more different substances. Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chem ...
... Characteristics of a substance that are observed when it reacts (changes) to produce one or more different substances. Example- Water can be changed into hydrogen gas and oxygen gas using an electric current. When water molecules change chemically into hydrogen gas and oxygen gas, we say that a chem ...
Modified ketone resin as an epoxy resin curing agent
... The C,H and Br contents of BCHF also agree with the predicted structure. The bromine content of BCHF polymer indicate that the bromination of terminal OH group could not be exist. This is only possible at higher temperature [11]. The reaction of BCHF with hydrazine and its various derivatives was ca ...
... The C,H and Br contents of BCHF also agree with the predicted structure. The bromine content of BCHF polymer indicate that the bromination of terminal OH group could not be exist. This is only possible at higher temperature [11]. The reaction of BCHF with hydrazine and its various derivatives was ca ...
Chemistry Content Review Notes
... (VDOE) Curriculum Framework, Enhanced Scope and Sequence, and Released Test items. In addition to VDOE information, Glencoe Textbook Series and resources have been used. Finally, information from various websites is included. The websites are listed with the information as it appears in the document ...
... (VDOE) Curriculum Framework, Enhanced Scope and Sequence, and Released Test items. In addition to VDOE information, Glencoe Textbook Series and resources have been used. Finally, information from various websites is included. The websites are listed with the information as it appears in the document ...
Answers to Selected Exercises
... (b) ¢H = 0 for mixing ideal gases. ¢S is positive, because the disorder of the system increases. (c) The process is spontaneous and therefore irreversible. (d) Since ¢H = 0, the process does not affect the entropy of the surroundings. 4.7 (a) At 300 K, ¢H = T¢S, ¢G = 0 and the system is at equilibri ...
... (b) ¢H = 0 for mixing ideal gases. ¢S is positive, because the disorder of the system increases. (c) The process is spontaneous and therefore irreversible. (d) Since ¢H = 0, the process does not affect the entropy of the surroundings. 4.7 (a) At 300 K, ¢H = T¢S, ¢G = 0 and the system is at equilibri ...
Stoichiometry, Lab Basics, Reactions
... ____ 19. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? A) It is equal to 1/3 of the total pressure. B) It depends on ...
... ____ 19. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? A) It is equal to 1/3 of the total pressure. B) It depends on ...
Notes for Quarter I
... fact that things move on the water’s surface that tells you that the waves are transferring energy. Most waves transfer energy by the vibration of particles in a medium – a substance through which a wave can travel. A medium (plural media) can be a solid, liquid, or gas. When a particle vibrates (mo ...
... fact that things move on the water’s surface that tells you that the waves are transferring energy. Most waves transfer energy by the vibration of particles in a medium – a substance through which a wave can travel. A medium (plural media) can be a solid, liquid, or gas. When a particle vibrates (mo ...
Evidence for the Predominance of Condensed Phase Reaction in
... gas phase O2 is released from Bi2O3.10 Since the system is reacting without the presence of a gas phase oxidizer, this suggests that there is condensed phase chemistry occurring. We also performed a high heating rate SEM study, which allowed for in situ heating of aluminum/metal oxide mixtures and i ...
... gas phase O2 is released from Bi2O3.10 Since the system is reacting without the presence of a gas phase oxidizer, this suggests that there is condensed phase chemistry occurring. We also performed a high heating rate SEM study, which allowed for in situ heating of aluminum/metal oxide mixtures and i ...
One-Pot Catalytic Conversion of Cellulose and of Woody
... found for the latter substrate (Table 1). In each case, a H2, CO, CO2, CH4 gas mixture was generated while the liquid fraction contained HAE and CAE in a ratio consistent with cellulose/ hemicellulose and lignin origins, respectively. The reaction time required for solubilization of the biomass soli ...
... found for the latter substrate (Table 1). In each case, a H2, CO, CO2, CH4 gas mixture was generated while the liquid fraction contained HAE and CAE in a ratio consistent with cellulose/ hemicellulose and lignin origins, respectively. The reaction time required for solubilization of the biomass soli ...
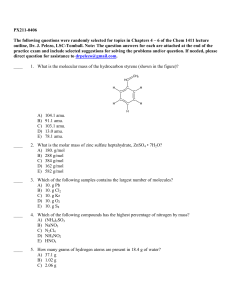
PX211-0406 The following questions were randomly selected for
... The following questions were randomly selected for topics in Chapters 4 – 6 of the Chem 1411 lecture outline, Dr. J. Pelezo, LSC-Tomball. Note: The question answers for each are attached at the end of the practice exam and include selected suggestions for solving the problems and/or question. If nee ...
... The following questions were randomly selected for topics in Chapters 4 – 6 of the Chem 1411 lecture outline, Dr. J. Pelezo, LSC-Tomball. Note: The question answers for each are attached at the end of the practice exam and include selected suggestions for solving the problems and/or question. If nee ...
Thermochemistry - Pearson Canada
... with which the system interacts. Figure 7-1 pictures three common systems: first, as we see them and, then, in an abstract form that chemists commonly use. An open system freely exchanges energy and matter with its surroundings (Fig. 7-1a). A closed system can exchange energy, but not matter, with i ...
... with which the system interacts. Figure 7-1 pictures three common systems: first, as we see them and, then, in an abstract form that chemists commonly use. An open system freely exchanges energy and matter with its surroundings (Fig. 7-1a). A closed system can exchange energy, but not matter, with i ...
File - IB CHEM NINJA
... rate of reaction (b) with time in establishing a chemical equilibrium In an equilibrium all of the species involved, both reactants and products, are present at a constant concentration. As a consequence, macroscopic properties of the system (that is those that can be observed or measured, such as i ...
... rate of reaction (b) with time in establishing a chemical equilibrium In an equilibrium all of the species involved, both reactants and products, are present at a constant concentration. As a consequence, macroscopic properties of the system (that is those that can be observed or measured, such as i ...
Handout on Buffer Solutions
... “Self, why don’t I just plug the concentration of NaA and HA into the H-H equation and solve for pH? Why do all this work?” Although the H-H equation is always valid, the [A-] and [HA] in the H-H equation are the equilibrium concentrations. Once you know [A-] and [HA] at equilibrium, you can plug th ...
... “Self, why don’t I just plug the concentration of NaA and HA into the H-H equation and solve for pH? Why do all this work?” Although the H-H equation is always valid, the [A-] and [HA] in the H-H equation are the equilibrium concentrations. Once you know [A-] and [HA] at equilibrium, you can plug th ...
an introduction to the concept of exergy and energy quality
... The main objective of this document is to serve as the reading text for the Exergy topic in the course Engineering Thermodynamics 1 provided by the Department of Energy and Process Engineering at the Norwegian University of Science and Technology, Trondheim, Norway. As such, it will conform as much ...
... The main objective of this document is to serve as the reading text for the Exergy topic in the course Engineering Thermodynamics 1 provided by the Department of Energy and Process Engineering at the Norwegian University of Science and Technology, Trondheim, Norway. As such, it will conform as much ...
File - Garbally Chemistry
... Always only ONE MOLE of what you are burning on the LHS of the equation To aid balancing the equation, remember... you get one carbon dioxide molecule for every carbon atom in the original and one water molecule for every two hydrogen atoms When you have done this, go back and balance the oxygen. ...
... Always only ONE MOLE of what you are burning on the LHS of the equation To aid balancing the equation, remember... you get one carbon dioxide molecule for every carbon atom in the original and one water molecule for every two hydrogen atoms When you have done this, go back and balance the oxygen. ...
General chemistry laboratory activities, Lorentz
... ÷ When is heated is preferable to tilting it slightly to avoid sudden release of vapours; Laboratory flasks are vessels (containers) which in laboratory and other scientific settings, they are usually referred to simply as flasks. Flasks come in a number of shapes and a wide range of sizes which are ...
... ÷ When is heated is preferable to tilting it slightly to avoid sudden release of vapours; Laboratory flasks are vessels (containers) which in laboratory and other scientific settings, they are usually referred to simply as flasks. Flasks come in a number of shapes and a wide range of sizes which are ...
Industrial Chemistry - Osun State University
... macromolecular structures. Lattice energy and forces between the particles in atomic molecular and ionic lattice. Electrolysis: The factors affecting the mass of substance liberated during electrolysis. Relationship between Faraday and the Avogadro constant and the charge of the electron. Equilibri ...
... macromolecular structures. Lattice energy and forces between the particles in atomic molecular and ionic lattice. Electrolysis: The factors affecting the mass of substance liberated during electrolysis. Relationship between Faraday and the Avogadro constant and the charge of the electron. Equilibri ...
PRACTICE – Naming and Writing Ionic Compounds
... Na2S2O3(aq) + 4Cl2(g) + 5H2O(aq) 2NaHSO4(aq) + 8HCl(aq) a. How many moles of Na2S2O3 are needed to react with 0.12mol of Cl2? ...
... Na2S2O3(aq) + 4Cl2(g) + 5H2O(aq) 2NaHSO4(aq) + 8HCl(aq) a. How many moles of Na2S2O3 are needed to react with 0.12mol of Cl2? ...
(MDCAT) 2017 - University Of Health Sciences Lahore
... terms of proton number and nucleon number. ii) Distinguish between isotopes on the basis of different numbers of neutrons present. f) Describe the number and relative energies of the s, p and d orbitals for the principal quantum numbers 1, 2 and 3 and also the 4s and 4p orbitals. g) Describe the sha ...
... terms of proton number and nucleon number. ii) Distinguish between isotopes on the basis of different numbers of neutrons present. f) Describe the number and relative energies of the s, p and d orbitals for the principal quantum numbers 1, 2 and 3 and also the 4s and 4p orbitals. g) Describe the sha ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.