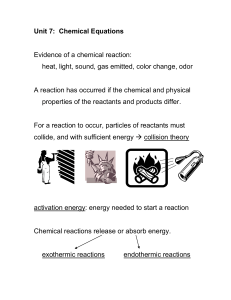
File - chemistryattweed
... and was interested in the effect of heat on the chemistry of gases. In the early 1900s, Haber reacted nitrogen with hydrogen, using an iron catalyst, to form ammonia. Ammonia can be readily converted to a range of valuable products. In 1908 he had improved the reaction and in 1911 he was rewarded wi ...
... and was interested in the effect of heat on the chemistry of gases. In the early 1900s, Haber reacted nitrogen with hydrogen, using an iron catalyst, to form ammonia. Ammonia can be readily converted to a range of valuable products. In 1908 he had improved the reaction and in 1911 he was rewarded wi ...
Science Focus 9 Matter and Chemical Change Class Notes Topic 1
... as elements. Lavoisier was one of the first chemists to use a balanced view of chemical change, which we now call … The Law of Conservation of Mass In a chemical reaction, the total mass of the reactants, is always equal to the total mass of the products. This law ties in well with the atomic theory ...
... as elements. Lavoisier was one of the first chemists to use a balanced view of chemical change, which we now call … The Law of Conservation of Mass In a chemical reaction, the total mass of the reactants, is always equal to the total mass of the products. This law ties in well with the atomic theory ...
Basic Concepts of the Gas Phase
... Alfons Buekens was born in Aalst, Belgium; he obtained his M.Sc. (1964) and his Ph.D (1967) at Ghent University (RUG) and received the K.V.I.V.-Award (1965), the Robert De Keyser Award (Belgian Shell Co., 1968), the Körber Foundation Award (1988) and the Coca Cola Foundation Award (1989). Dr. Bueken ...
... Alfons Buekens was born in Aalst, Belgium; he obtained his M.Sc. (1964) and his Ph.D (1967) at Ghent University (RUG) and received the K.V.I.V.-Award (1965), the Robert De Keyser Award (Belgian Shell Co., 1968), the Körber Foundation Award (1988) and the Coca Cola Foundation Award (1989). Dr. Bueken ...
Energy - Effingham County Schools
... Heat – the flow of energy from an object at a higher temperature to an object at a lower temperature. ...
... Heat – the flow of energy from an object at a higher temperature to an object at a lower temperature. ...
AIPMT Syllabus
... Lussac’s law, Avogadro’s law, ideal behaviour of gases, empirical derivation of gas equation. Avogadro number, ideal gas equation. Kinetic energy and molecular speeds (elementary idea), deviation from ideal behaviour, liquefaction of gases, critical temperature. ...
... Lussac’s law, Avogadro’s law, ideal behaviour of gases, empirical derivation of gas equation. Avogadro number, ideal gas equation. Kinetic energy and molecular speeds (elementary idea), deviation from ideal behaviour, liquefaction of gases, critical temperature. ...
ppt
... Steps in using bond energies to predict ∆H for a reaction 1. Determine how many of each type of bond must be broken in the reactants. 2. Determine the number of bonds of each type that form in the products. 3. Use bond energy data from Table 1 (page 307) to calculate the total energy required to bre ...
... Steps in using bond energies to predict ∆H for a reaction 1. Determine how many of each type of bond must be broken in the reactants. 2. Determine the number of bonds of each type that form in the products. 3. Use bond energy data from Table 1 (page 307) to calculate the total energy required to bre ...
Thermochem ppt
... Σ (sigma) means “the sum of,” n is the amount (in moles) of a particular bond type, and D is the bond energy per mole of bonds. D is obtained from reference tables Steps in using bond energies to predict ∆H for a reaction 1. Determine how many of each type of bond must be broken in the reactants. 2 ...
... Σ (sigma) means “the sum of,” n is the amount (in moles) of a particular bond type, and D is the bond energy per mole of bonds. D is obtained from reference tables Steps in using bond energies to predict ∆H for a reaction 1. Determine how many of each type of bond must be broken in the reactants. 2 ...
Chapter 9: Energy and Chemistry
... • Phase changes occur under constant pressure conditions. – The heat flow during a phase change is an enthalpy change. – During a phase change, temperature does not change with heat flow due to formation or breaking of intermolecular attractive forces. ...
... • Phase changes occur under constant pressure conditions. – The heat flow during a phase change is an enthalpy change. – During a phase change, temperature does not change with heat flow due to formation or breaking of intermolecular attractive forces. ...
Slide 1
... As the oxygen atoms are shared between adjacent tetrahedra, the chemical formula is SiO3 or Si2O6. MgSiO3 or Mg2Si2O6 (enstatite, as compared to forsterite there is ...
... As the oxygen atoms are shared between adjacent tetrahedra, the chemical formula is SiO3 or Si2O6. MgSiO3 or Mg2Si2O6 (enstatite, as compared to forsterite there is ...
AIPMT prelims examination last year cut off
... elucidating the concept of the molecule, Boyle’s law, Charle’s law, Gay Lussac’s law, Avogadro’s law, ideal behaviour of gases, empirical derivation of gas equation. Avogadro number, ideal gas equation. Kinetic energy and molecular speeds (elementary idea), deviation from ideal behaviour, liquefacti ...
... elucidating the concept of the molecule, Boyle’s law, Charle’s law, Gay Lussac’s law, Avogadro’s law, ideal behaviour of gases, empirical derivation of gas equation. Avogadro number, ideal gas equation. Kinetic energy and molecular speeds (elementary idea), deviation from ideal behaviour, liquefacti ...
MS-Physical - Science AZ
... Alignment with National Science Standards Use the chart below to find Science A-Z units that best support the Next Generation Science Standards* for Middle School Physical Science, and several featured resources from those units that provide strong connections. Each Performance Expectation in the ch ...
... Alignment with National Science Standards Use the chart below to find Science A-Z units that best support the Next Generation Science Standards* for Middle School Physical Science, and several featured resources from those units that provide strong connections. Each Performance Expectation in the ch ...
malhotra book depot
... What is AZT ? Give its use. AZT is Azidothymidine. It is used for AIDS victims. Name two chemical compounds which are used in the treatment of cancer. Cis-platin and taxol. Name the compound which is generally added for the sterilization of water. Chlorine (0.2 to 0.4 ppm). ...
... What is AZT ? Give its use. AZT is Azidothymidine. It is used for AIDS victims. Name two chemical compounds which are used in the treatment of cancer. Cis-platin and taxol. Name the compound which is generally added for the sterilization of water. Chlorine (0.2 to 0.4 ppm). ...
Strumenti tutor LIM
... In a physical transformation...................... (no change takes place in the identity of the substance) A chemical transformation takes place when....................(atoms in the reactants are rearranged to form new substabces)(old bonds are broken and new bonds are formed)( at least one new su ...
... In a physical transformation...................... (no change takes place in the identity of the substance) A chemical transformation takes place when....................(atoms in the reactants are rearranged to form new substabces)(old bonds are broken and new bonds are formed)( at least one new su ...
CHEMISTRY 1710 - Practice Exam #2
... _____17. Which of the following substances (with specific heat capacity provided) would show the greatest temperature change upon absorbing 100.0 J of heat? A) 10.0 g Fe, CFe = 0.449 J/g°C B) 10.0 g H2O, CH2O = 4.18 J/g°C C) 10.0 g ethanol, Cethanol = 2.42 J/g°C D) 10.0 g Au, CAu = 0.128 J/g°C ____ ...
... _____17. Which of the following substances (with specific heat capacity provided) would show the greatest temperature change upon absorbing 100.0 J of heat? A) 10.0 g Fe, CFe = 0.449 J/g°C B) 10.0 g H2O, CH2O = 4.18 J/g°C C) 10.0 g ethanol, Cethanol = 2.42 J/g°C D) 10.0 g Au, CAu = 0.128 J/g°C ____ ...
Practice Test 11 - U of L Class Index
... A chunk of white phosphorus weighing 6.58 grams is put in a 750 mL flask containing dry argon (which is then removed using a vacuum, leaving only the phosphorus in the flask). A separate 750 mL flask contains 3.15 bar of fluorine gas (at 19.65 °C). The two flasks are connected so that the two compou ...
... A chunk of white phosphorus weighing 6.58 grams is put in a 750 mL flask containing dry argon (which is then removed using a vacuum, leaving only the phosphorus in the flask). A separate 750 mL flask contains 3.15 bar of fluorine gas (at 19.65 °C). The two flasks are connected so that the two compou ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.