Phase, Q, Curves
... Thermal Chemistry Phase: any part of a system that has uniform composition and properties. ( solid, liquid, gas) Overhead ...
... Thermal Chemistry Phase: any part of a system that has uniform composition and properties. ( solid, liquid, gas) Overhead ...
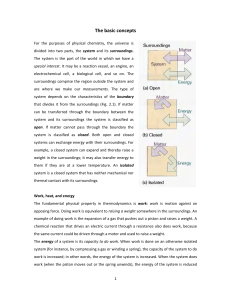
The basic concepts For the purposes of physical chemistry, the
... The concept of temperature springs from the observation that a change in physical state (for example, a change of volume) can occur when two objects are in contact with one another, as when a red-hot metal is plunged into water. Later we shall see that the change in state can be interpreted as arisi ...
... The concept of temperature springs from the observation that a change in physical state (for example, a change of volume) can occur when two objects are in contact with one another, as when a red-hot metal is plunged into water. Later we shall see that the change in state can be interpreted as arisi ...
Powerpoint Point
... whereas things like wood or plastics are not good conductors of heat. Those that are not so good conductors are called insulators. ...
... whereas things like wood or plastics are not good conductors of heat. Those that are not so good conductors are called insulators. ...
CHAPTER 10 INTRODUCTION TO COMPRESSIBLE FLOW
... level, there are 100 possible microstates: Ω = 100. When the same amount of internal energy is added again, there are 100 × 100 possible states (9900 states with two molecules up one level and 100 states with one molecule up two levels): Ω = 10, 000. The entropy increases as the internal energy incr ...
... level, there are 100 possible microstates: Ω = 100. When the same amount of internal energy is added again, there are 100 × 100 possible states (9900 states with two molecules up one level and 100 states with one molecule up two levels): Ω = 10, 000. The entropy increases as the internal energy incr ...
Extra Unit 3 Problems for the Web Site (Honors
... The following problems are for Honors only: 9. When 4.90 g of KClO3 was heated, it showed a mass loss of 0.384 g. Find the percent of the original KClO3 that had decomposed. 10. When 10.0 g of silicon dust, Si, is exploded with 100.0 g of oxygen, O2, forming silicon dioxide, SiO2, how many grams of ...
... The following problems are for Honors only: 9. When 4.90 g of KClO3 was heated, it showed a mass loss of 0.384 g. Find the percent of the original KClO3 that had decomposed. 10. When 10.0 g of silicon dust, Si, is exploded with 100.0 g of oxygen, O2, forming silicon dioxide, SiO2, how many grams of ...
$doc.title
... mutagenicity or Ames test. The Ames test assesses the ability of a chemical to induce mutations in any of several different strains of bacteria. A positive test in any strain indicates the chemical is ...
... mutagenicity or Ames test. The Ames test assesses the ability of a chemical to induce mutations in any of several different strains of bacteria. A positive test in any strain indicates the chemical is ...
PHYSICAL SETTING CHEMISTRY
... cell, using the smallest whole-number coefficients. [1] 64 Identify one metal from Table J that is more easily oxidized than Zn. [1] 65 Explain, in terms of Zn atoms and Zn ions, why the mass of the Zn electrode decreases as the cell operates. [1] ...
... cell, using the smallest whole-number coefficients. [1] 64 Identify one metal from Table J that is more easily oxidized than Zn. [1] 65 Explain, in terms of Zn atoms and Zn ions, why the mass of the Zn electrode decreases as the cell operates. [1] ...
ap-thermochemistry - Waukee Community School District Blogs
... Hess’s Law Enthalpy changes are state functions. It does not matter if ΔH for a reaction is calculated in one step or a series of steps = Hess’s Law ...
... Hess’s Law Enthalpy changes are state functions. It does not matter if ΔH for a reaction is calculated in one step or a series of steps = Hess’s Law ...
Chapter 3
... • Methionine, an amino acid used by organisms to make proteins, is represented below. Write the formula for methionine and calculate its molar mass. (red = O; gray = C; blue = N; yellow = S; ivory = H) ...
... • Methionine, an amino acid used by organisms to make proteins, is represented below. Write the formula for methionine and calculate its molar mass. (red = O; gray = C; blue = N; yellow = S; ivory = H) ...
Work Done On or By a Gas
... • Step 1: Gas in the system absorbs heat (Qin) from a heat source at a high temperature Th, expands and does work on surroundings without increasing internal energy • Step 2: The gas expands quickly and does work on surroundings, which causes the system to cool to a lower temperature, Tc. • Step 3: ...
... • Step 1: Gas in the system absorbs heat (Qin) from a heat source at a high temperature Th, expands and does work on surroundings without increasing internal energy • Step 2: The gas expands quickly and does work on surroundings, which causes the system to cool to a lower temperature, Tc. • Step 3: ...
Chemical Reactions Chemistry - is the study of matter, its properties
... How can we check the properties of unknown chemicals to understand or predict the reaction with another chemical? Many chemicals can be hazardous to human health or the environment if they are not handled safely. There are a variety of symbols used to identify hazardous chemicals. Many household pro ...
... How can we check the properties of unknown chemicals to understand or predict the reaction with another chemical? Many chemicals can be hazardous to human health or the environment if they are not handled safely. There are a variety of symbols used to identify hazardous chemicals. Many household pro ...
File
... 1) How many different substances are described on the “left side” of the equation? 2) How many different substances described on the “right side” of the equation? 3) What does this tell me? I can look at an equation to see if a change is chemical or physical. In this case, since a new substance is f ...
... 1) How many different substances are described on the “left side” of the equation? 2) How many different substances described on the “right side” of the equation? 3) What does this tell me? I can look at an equation to see if a change is chemical or physical. In this case, since a new substance is f ...
Notes
... or identity of a substance • Physical change - produces a recognizable difference in the appearance of a substance without causing any change in its composition or identity - conversion from one physical state to another - melting an ice cube ...
... or identity of a substance • Physical change - produces a recognizable difference in the appearance of a substance without causing any change in its composition or identity - conversion from one physical state to another - melting an ice cube ...
C. - Knights of The Periodic Table
... created the periodic table shown. The astronaut was trying to determine what type of bond would be present in several compounds. The type of bond in a compound containing G and E would be — ...
... created the periodic table shown. The astronaut was trying to determine what type of bond would be present in several compounds. The type of bond in a compound containing G and E would be — ...
Enthalpy In A Box: Teaching Open Vs. Closed System Work Terms
... Enthalpy, somewhat like entropy appears to be one of thermodynamic’s mysterious and abstract properties due primarily to difficulty in physical comprehension. Unlike entropy however, one could argue that enthalpy does not bring as much to the table. After all, it is defined, somewhat arbitrarily, fr ...
... Enthalpy, somewhat like entropy appears to be one of thermodynamic’s mysterious and abstract properties due primarily to difficulty in physical comprehension. Unlike entropy however, one could argue that enthalpy does not bring as much to the table. After all, it is defined, somewhat arbitrarily, fr ...
chapter 1 - College Test bank - get test bank and solution manual
... liquid gasoline is converted to heat and gases. Another constructive example is the burning of coal to heat water into steam, which is then used to turn a turbine and produce electricity. The combustion of coal results in a flame plus other gases. The above two examples are examples of chemical chan ...
... liquid gasoline is converted to heat and gases. Another constructive example is the burning of coal to heat water into steam, which is then used to turn a turbine and produce electricity. The combustion of coal results in a flame plus other gases. The above two examples are examples of chemical chan ...
PHYSICAL SETTING CHEMISTRY
... A separate answer sheet for Part A and Part B–1 has been provided to you. Follow the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers ...
... A separate answer sheet for Part A and Part B–1 has been provided to you. Follow the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers ...
Practice Test 1 (Chapters 1-7)
... the smallest possible integers, what is the number in front of the substance in bold type? NBr3 + NaOH N2 + NaBr + HOBr a. b. c. d. e. ...
... the smallest possible integers, what is the number in front of the substance in bold type? NBr3 + NaOH N2 + NaBr + HOBr a. b. c. d. e. ...
File - SRIT - MECHANICAL ENGINEERING
... So far, no attempt has been made to relate these interactions between themselves and with the energy content of the system. First law of thermodynamics, often called as law of conservation of energy, relating work, heat, and energy content of the system will be discussed in detail in this chapter. ...
... So far, no attempt has been made to relate these interactions between themselves and with the energy content of the system. First law of thermodynamics, often called as law of conservation of energy, relating work, heat, and energy content of the system will be discussed in detail in this chapter. ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.