Energy (eV) - Integrated Composites Lab
... for both Cu and Co, it sloWs the displacement reaction via an adsorbed intermediate, and it buffers the electrode (Co/ Cu) surface. Although a complexing agent such as citrate is preferred, its presence may not be crucial to the functioning of the invention. Other retarding agents may also be used, ...
... for both Cu and Co, it sloWs the displacement reaction via an adsorbed intermediate, and it buffers the electrode (Co/ Cu) surface. Although a complexing agent such as citrate is preferred, its presence may not be crucial to the functioning of the invention. Other retarding agents may also be used, ...
Unit 10 complete 2016-2017
... Moles of A to Moles of B Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water ...
... Moles of A to Moles of B Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water ...
Honors Chemistry
... Moles of A to Moles of B Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water ...
... Moles of A to Moles of B Work the following out on a separate sheet of paper. 1. Hydrogen and oxygen react under certain conditions to product water. a. How many moles of hydrogen would be needed to produce 5.0 moles of water? b. How many moles of oxygen would be needed to produce 5.0 moles of water ...
Massachusetts Tests for Educator Licensure (MTEL )
... Correct Response: D. The combination of chemicals is that of a weak acid and a strong base. This conclusion can be drawn because the equivalence point on the graph corresponds to a pH greater than 7. It is clear that a weak acid is being titrated with a strong base (instead of a strong base being ti ...
... Correct Response: D. The combination of chemicals is that of a weak acid and a strong base. This conclusion can be drawn because the equivalence point on the graph corresponds to a pH greater than 7. It is clear that a weak acid is being titrated with a strong base (instead of a strong base being ti ...
Now! - Soojeede.com
... (b) NaOH has Na as a cation, not H (or starts with a cation other than H ) and is therefore not an acid. By writing the dissociation equation we see that NaOH is definitely not an acid. ...
... (b) NaOH has Na as a cation, not H (or starts with a cation other than H ) and is therefore not an acid. By writing the dissociation equation we see that NaOH is definitely not an acid. ...
Exam 2 Key
... electricity. A strongly electyroytic solution conducts electricity well because it contains a high concentration of ions, while a weakly electrolytic solution is a poor conductor because of a low concentration of ions. 4. Complete the following table. (12 points) Oxidation States ...
... electricity. A strongly electyroytic solution conducts electricity well because it contains a high concentration of ions, while a weakly electrolytic solution is a poor conductor because of a low concentration of ions. 4. Complete the following table. (12 points) Oxidation States ...
Mole-Volume Conversion Assignment
... Yesterday’s calculations we found out that when we use 50mL of 5% acetic acid solutions, we require 3.5g of sodium bicarbonate to completely react. Trial 1: use 1.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left over: NaHCO3 all used Trial 2: use 2.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left ...
... Yesterday’s calculations we found out that when we use 50mL of 5% acetic acid solutions, we require 3.5g of sodium bicarbonate to completely react. Trial 1: use 1.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left over: NaHCO3 all used Trial 2: use 2.0g of NaHCO3 and 50mL CH3COOH: extra vinegar left ...
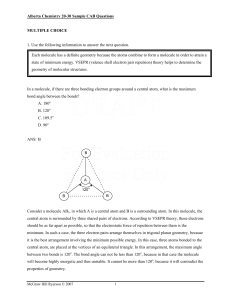
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
Analytical Chemistry - University of Delhi
... 1. Estimation of sodium carbonate and sodium hydrogen carbonate present in a mixture. 2. Estimation of oxalic acid by titrating it with KMnO4. 3. Estimation of water of crystallization in Mohr’s salt by titrating with KMnO4. 4. Estimation of Fe (II) ions by titrating it with K2Cr2O7 using internal i ...
... 1. Estimation of sodium carbonate and sodium hydrogen carbonate present in a mixture. 2. Estimation of oxalic acid by titrating it with KMnO4. 3. Estimation of water of crystallization in Mohr’s salt by titrating with KMnO4. 4. Estimation of Fe (II) ions by titrating it with K2Cr2O7 using internal i ...
c00kieee - Ritter Illustration
... reactions lead to the formation of a variety of actinide elements, for example, Np, Pu, Am, and Cm, as radioactive fission products. As a result of the production of these highly radioactive elements, burnt nuclear fuel must be allowed to ‘cool’ until the short-lived isotopes decay away and reduce t ...
... reactions lead to the formation of a variety of actinide elements, for example, Np, Pu, Am, and Cm, as radioactive fission products. As a result of the production of these highly radioactive elements, burnt nuclear fuel must be allowed to ‘cool’ until the short-lived isotopes decay away and reduce t ...
ppt
... If the concentrations at one point in the reaction are: [CO(g)] = 4.00 mol/L, [H2O(g)] = 2.00 mol/L, [CO2(g)] = 4.00 mol/L, and [H2(g)] = 2.00 mol/L. Determine whether the reaction has reached equilibrium, and, if not, in which direction it will proceed to establish equilibrium. ...
... If the concentrations at one point in the reaction are: [CO(g)] = 4.00 mol/L, [H2O(g)] = 2.00 mol/L, [CO2(g)] = 4.00 mol/L, and [H2(g)] = 2.00 mol/L. Determine whether the reaction has reached equilibrium, and, if not, in which direction it will proceed to establish equilibrium. ...
IIT-JEE - Brilliant Public School Sitamarhi
... Point defects: When ions or atoms do not hold the theoretical position, this is called point defect. Point defects are of two types: Stoichiometric defects: Schottky defect: Due to missing of ions from lattice point in pairs. Frenkel defect: It is caused due to the creation of lattice vacancy as a r ...
... Point defects: When ions or atoms do not hold the theoretical position, this is called point defect. Point defects are of two types: Stoichiometric defects: Schottky defect: Due to missing of ions from lattice point in pairs. Frenkel defect: It is caused due to the creation of lattice vacancy as a r ...
Ch 10 Practice Problems 1. Consider the process A(l) A(s). Which
... C) greater than zero. D) More information is needed. q is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. H is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. E is A) less than zero. B) equal to zero. C) greater th ...
... C) greater than zero. D) More information is needed. q is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. H is A) less than zero. B) equal to zero. C) greater than zero. D) More information is needed. E is A) less than zero. B) equal to zero. C) greater th ...
Laboratory Manual
... 2. Make sure the hose for your Bunsen burner does not have cracks and holes where gas may leak out. After you are sure it is safe to use, connect one end to the Bunsen burner and the other to the gas source. 3. Turn on the gas and light the Bunsen burner by either using a striker or a flame source s ...
... 2. Make sure the hose for your Bunsen burner does not have cracks and holes where gas may leak out. After you are sure it is safe to use, connect one end to the Bunsen burner and the other to the gas source. 3. Turn on the gas and light the Bunsen burner by either using a striker or a flame source s ...
g - mrnicholsscience
... …that the one with more mass is in excess—you might need more of it. If 150 g nitrogen and 40 g hydrogen make ammonia… 8g hydrogen is left over …that the one with more moles is in excess—you might need more of it. If 5 moles oxygen and 8 moles hydrogen make water… 1 mol oxygen is left over ...
... …that the one with more mass is in excess—you might need more of it. If 150 g nitrogen and 40 g hydrogen make ammonia… 8g hydrogen is left over …that the one with more moles is in excess—you might need more of it. If 5 moles oxygen and 8 moles hydrogen make water… 1 mol oxygen is left over ...
Organic Chemistry
... yields, respectively. The structure of the prepared PS-DIB was confirmed by FTIR, which exhibited two strong bands at 1639 and 1712 cm-1 corresponding to the carbonyl group. The loading of the (diacetoxyiodo)phenyl group on polystyrene was determined by iodometric titration and calculated to be 1.39 ...
... yields, respectively. The structure of the prepared PS-DIB was confirmed by FTIR, which exhibited two strong bands at 1639 and 1712 cm-1 corresponding to the carbonyl group. The loading of the (diacetoxyiodo)phenyl group on polystyrene was determined by iodometric titration and calculated to be 1.39 ...
Amines - ncert
... The lower aliphatic amines are gases with fishy odour. Primary amines with three or more carbon atoms are liquid and still higher ones are solid. Aniline and other arylamines are usually colourless but get coloured on storage due to atmospheric oxidation. Lower aliphatic amines are soluble in water ...
... The lower aliphatic amines are gases with fishy odour. Primary amines with three or more carbon atoms are liquid and still higher ones are solid. Aniline and other arylamines are usually colourless but get coloured on storage due to atmospheric oxidation. Lower aliphatic amines are soluble in water ...
Equilibrium Booklet - mrstorie
... 3. Chemists have determined the equilibrium constants for several reactions. In which of these reactions are the products favoured over the reactants? a. KC = 1.0×102 ...
... 3. Chemists have determined the equilibrium constants for several reactions. In which of these reactions are the products favoured over the reactants? a. KC = 1.0×102 ...
Kinetic modelling of the Maillard reaction between proteins and sugars
... 1.3 Sugar degradation ...
... 1.3 Sugar degradation ...
Worked solutions to the problems
... This year we have 4 detailed preparatory laboratory exercises that cover the skills that your students need to show in Melbourne. We have tried to highlight the procedures in each exercise that need some particular caution, even for students of Olympiad level but our warnings cannot be comprehensive ...
... This year we have 4 detailed preparatory laboratory exercises that cover the skills that your students need to show in Melbourne. We have tried to highlight the procedures in each exercise that need some particular caution, even for students of Olympiad level but our warnings cannot be comprehensive ...
Wilhelm Ostwald, the Father of Physical Chemistry
... all concentrations where interionic forces cannot be neglected. The explanation for a decrease in conductivity with increase in concentration is given by the Debye— Hückel—Onsager theory. According to this theory, there are two factors that affect conductivity: (i) Each ion will be surrounded by an ...
... all concentrations where interionic forces cannot be neglected. The explanation for a decrease in conductivity with increase in concentration is given by the Debye— Hückel—Onsager theory. According to this theory, there are two factors that affect conductivity: (i) Each ion will be surrounded by an ...
Η - Knockhardy
... the strength of a bond depends on its environment so MEAN values are quoted making a bond is an exothermic process as it is the opposite of breaking a bond for diatomic gases, the bond enthalpy is twice the enthalpy of atomisation the smaller the bond enthalpy, the weaker the bond and the easier it ...
... the strength of a bond depends on its environment so MEAN values are quoted making a bond is an exothermic process as it is the opposite of breaking a bond for diatomic gases, the bond enthalpy is twice the enthalpy of atomisation the smaller the bond enthalpy, the weaker the bond and the easier it ...
From Organometallic Zinc and Copper Complexes to Highly
... since the organo-zinc carboxylate cluster compound (Et4Zn5(stearate)6) was successfully detected, a compound which is known to hydrolyze readily in the presence moisture.14b, 14d Furthermore, the zinc carbon bond lengths determined by the fits to the EXAFS data are in good agreement with those obtai ...
... since the organo-zinc carboxylate cluster compound (Et4Zn5(stearate)6) was successfully detected, a compound which is known to hydrolyze readily in the presence moisture.14b, 14d Furthermore, the zinc carbon bond lengths determined by the fits to the EXAFS data are in good agreement with those obtai ...
Chapter 3: Stoichiometry
... Decomposition Reactions A single reactant breaks into two or more products ...
... Decomposition Reactions A single reactant breaks into two or more products ...
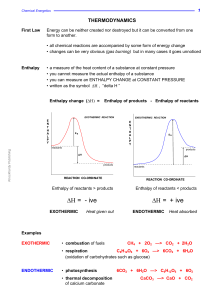
Redox

Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between species. The term ""redox"" comes from two concepts involved with electron transfer: reduction and oxidation. It can be explained in simple terms: Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, these are only specific examples of a more general concept of reactions involving electron transfer.Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid–base reactions. Like acid–base reactions, redox reactions are a matched set, that is, there cannot be an oxidation reaction without a reduction reaction happening simultaneously. The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction. When writing half-reactions, the gained or lost electrons are typically included explicitly in order that the half-reaction be balanced with respect to electric charge.Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation and reduction properly refer to a change in oxidation state — the actual transfer of electrons may never occur. The oxidation state of an atom is the fictitious charge that an atom would have if all bonds between atoms of different elements were 100% ionic. Thus, oxidation is better defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as ""redox"" even though no electron transfer occurs (such as those involving covalent bonds).There are simple redox processes, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), and more complex processes such as the oxidation of glucose (C6H12O6) in the human body through a series of complex electron transfer processes.